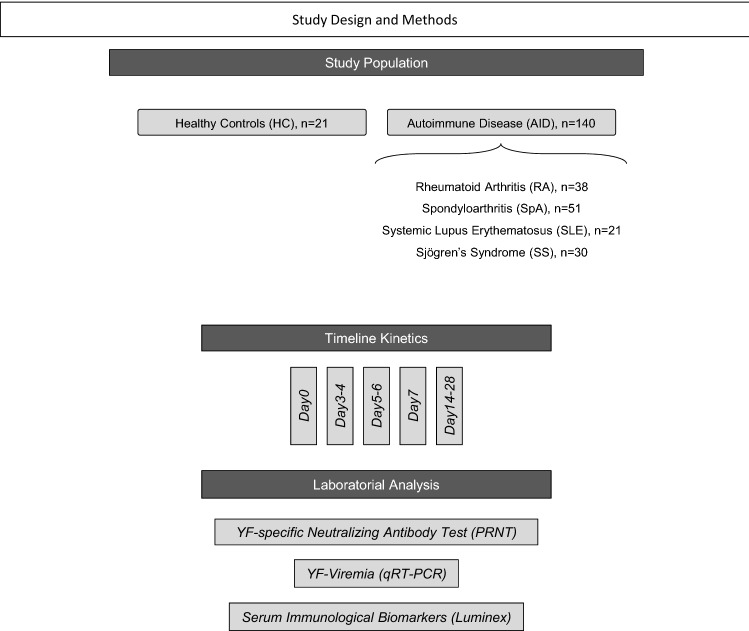
Figure 7.
Study Design and Methods. This is a prospective non-interventional observational study carried out between March, 2017 and July, 2017 in Vitória, Espírito Santo, Brazil. A total of 140 patients with Autoimmune Disease patients (AID) including: Rheumatoid Arthritis (RA, n = 38), Spondyloarthritis (SpA, n = 51), Systemic Lupus Erythematosus (SLE, n = 21) and Sjögren’s Syndrome (SS, n = 30). A group Healthy Controls without diagnosis of autoimmune diseases (HC, n = 21) was also included. All participants received the primary dose of 17DD-YF vaccine (Bio-Manguinhos-FIOCRUZ) during the 2017 Brazilian YF vaccination campaign. Detailed inclusion and exclusion criteria are provided in Methods. Blood samples were collected from each participant at distinct time points, including: D0; Day3–4; Day5–6; Day7; Day14–D28. Serum samples were used for laboratorial analysis, including: YF-specific neutralizing antibodies analysis by Plaque Reduction Neutralizing Test (PRNT), YF viral RNAnemia (YF-Viremia) detection by quantitative real time PCR (qRT-PCR) and Serum Immunological Biomarkers quantification by Luminex Bio-plex assay.