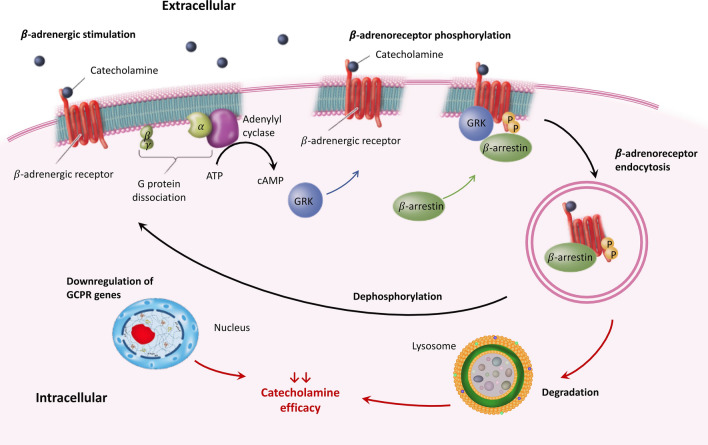
Fig. 2.
Mechanisms of adrenergic receptor activation and desensitization (here, β-adrenergic receptor). When catecholamines bind to G protein-coupled receptors (GPCR), there is a conformational change leading to dissociation of the receptors’ G protein subunits and transformation of ATP into cyclic adenosine monophosphate (cAMP). GPCR kinases (GRK) phosphorylate GPCR but only in their agonist-occupied receptor configuration. This GPCR phosphorylation by GRK enhances GPCR interaction with cytosolic proteins known as β-arrestins. These later bind to the GPCR and prevent further intracellular G-protein signaling, preventing the subsequent production of more cAMP. Increased GRK activity forces the equilibrium of β-receptors towards an inactive state. Moreover, β-arrestins promote GPCR internalization and their subsequent degradation in lysosomes. Resensitization is the process that restores the responsiveness of the desensitized receptors, through dephosphorylation by specific phosphatases and receptor recycling back to the cell membrane. Downregulation of genes encoding GPCRs also leads to reduced catecholamine efficacy, through a net loss of receptors. Of note, catecholamine binding modulates a complex cascade of enzymes and transmembrane ion channel activation, all of which are also prone to acute alteration and dysregulation due to sepsis [106–110]