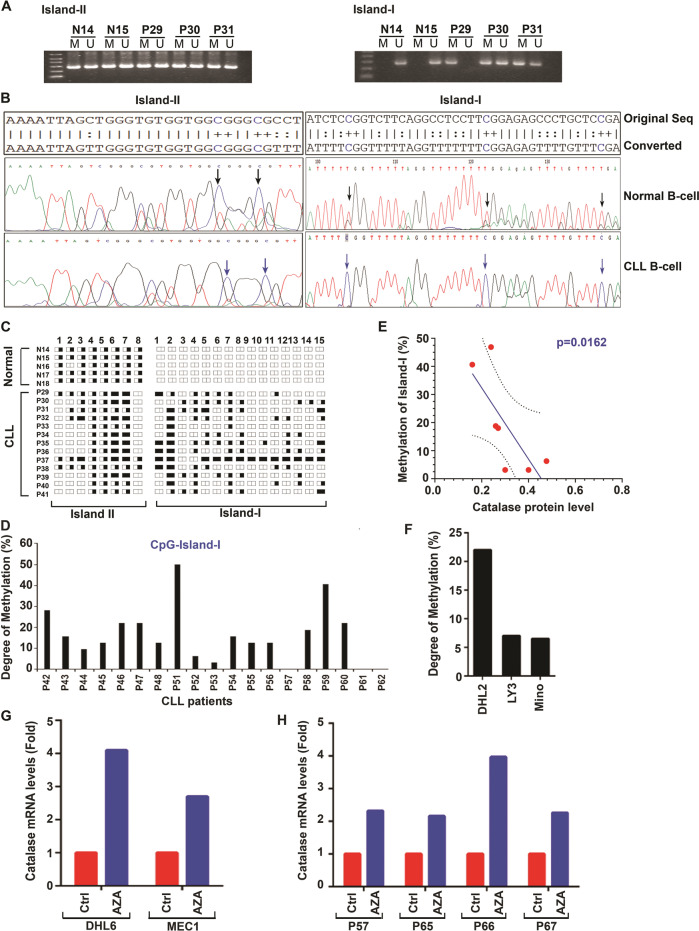
Fig. 4. Catalase expression is epigenetically regulated in CLL cells.
A Human catalase promoter possesses two distinct CpG-islands. Genomic DNA from normal B-cells (N14, N15) and CLL cells (P29–P31) were bisulfite converted and the predicted CpG-Islands in the catalase promoter were PCR amplified using methylation/unmethylation-specific (M/U) primer sets. PCR products were analyzed on agarose gels and photographed. B, C Methylation status in CpG-Island-I determines catalase expression in CLL cells. Genomic DNA from normal B-cells (n = 5; N14–N18) and CLL cells (n = 32; P29–P48, P51–P62) were bisulfite converted and the CpG-Island-I and -II in the catalase promoter were PCR amplified as described above and sequenced. Representative DNA sequence analyses from normal B-cells and CLL cells are shown and compared (B). A diagrammatic presentation of the methylation status in CpG-Island-I and –II of the catalase promoter in normal B-cells (n = 5; N14–N18) vs. CLL cells (n = 13; P29–P41) (obtained from the sequence analysis data) is shown in panel C. Each spliced rectangle (representing both alleles of the gene) indicates a putative CG-methylation site; half-filled rectangle represents methylation at one allele; filled rectangle represents methylation at both the alleles; empty rectangle indicates no methylation at either alleles. D CpG-Island-I in CLL cells is methylated differentially. Methylation status of the fifteen putative CpG sites in Island-I of the catalase promoter in CLL cells (n = 19; P42–P48, P51–P62) was determined by analyzing the sequence data described above and presented as “Degree of Methylation”. E Degree of Methylation in CpG-Island-I inversely correlates with catalase protein levels. CLL cell lysates as available from few CLL patients analyzed in panel D (P42–P45, P47, P48, P53) were examined for catalase expression in western blots. Normalized catalase protein levels (with respect to GAPDH) in CLL cells were further analyzed to find any correlation with the degree of methylation in CpG-Island-I of the catalase promoter. F Methylation status of CpG-Island-I in B-cell lymphoma cells. Similarly, degree of methylation in CpG-Island-I of the catalase promoter in other B-cell malignancies including diffuse large B-cell lymphoma (DHL2, LY3) and mantle cell lymphoma (Mino) was also determined. G, H Treatment of malignant B-cells with 5’-azacytidine increases catalase expression. DHL6/MEC1 cells (G) or primary CLL cells (H; n = 4; P57, P65–P67) were treated with a demethylating agent 5’-azacytidine (AZA; 1 µM) or left untreated. Catalase mRNA levels were determined by qRT-PCR from total RNA. GAPDH was used as internal control. Ct values were calculated and results are presented as “fold change” in treated vs. untreated cells.