FIGURE 1.
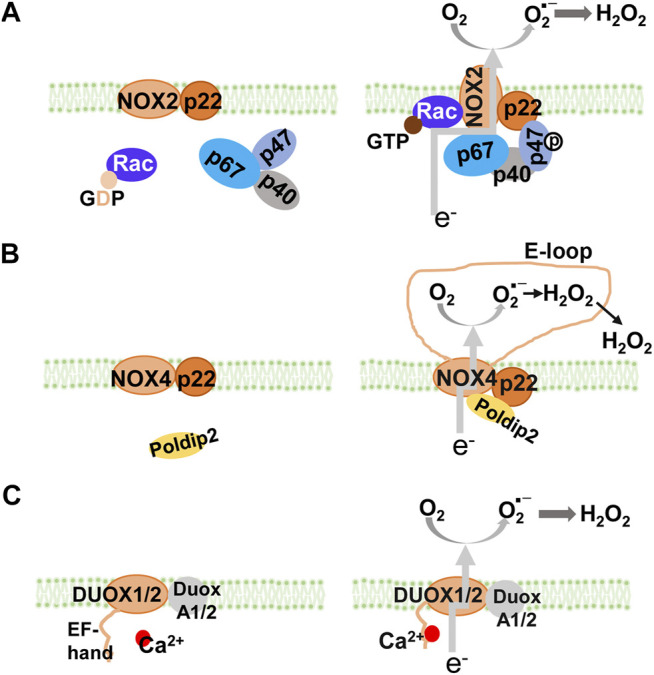
NADPH oxidase isoforms and their assembly. All NOX isoforms have membrane-spanning catalytic subunit that transfers electrons from NADPH through their NADPH and FAD-binding sites and heme groups to reduce O2 into superoxide anion (A) NOX1, NOX2, and NOX3 are cytosolic activators-dependent with the stabilizing p22phox membrane subunit. Activation of NOX2 requires assembly with cytosolic p47phox (equivalent to NoxO1 in NOX1 and NOX3), p67phox (equivalent to NoxA1 in NOX1 and NOX3), and p40phox. In resting cells (left panel), NOX2 and p22phox are associated, co-stabilizing each other. Upon activation (right panel), GDP is exchanged for GTP on Rac. The phosphorylation of p47phox subunit leads to a conformational change, which brings with p67phox and p40phox, to form the active NOX2 enzyme complex (NOX1 and NOX3 are not shown) (B) NOX4 is constitutively active with the stabilizing p22phox membrane subunit. Its activity can be modulated by Poldip2. Inside the E-loop (3rd extracellular loop), superoxide is rapidly dismutated into H2O2 (C) NOX5, DUOX1, and DUOX2 are Ca2+ dependent with EF-hand domains. DUOX1 and DUOX2 (as well as NOX5, not shown) have Ca2+ binding domains that undergo conformational change in response to an increase in intracellular Ca2+. It is speculated that activation of DUOX1, DUOX2, and NOX5, is through intramolecular protein-protein interaction between the Ca2+-binding domain and the C-terminal catalytic domain. GDP, guanosine diphosphate; GTP, guanosine-5′-triphosphate; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase.
