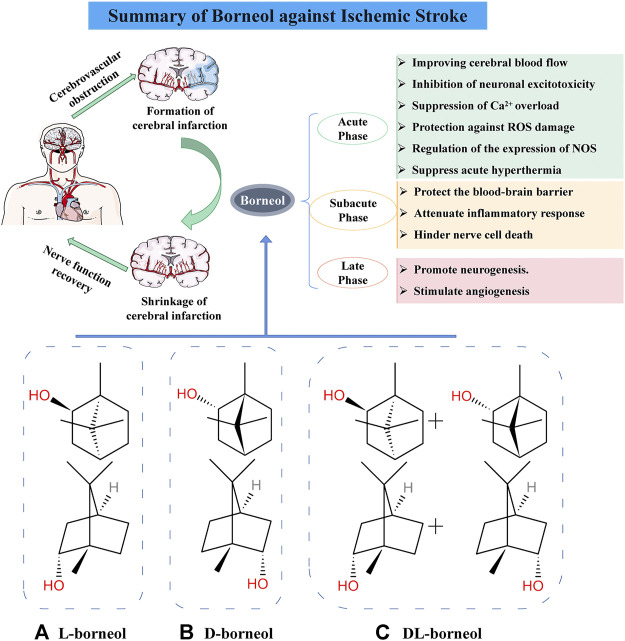
FIGURE 1.
Mechanism of borneol against cerebral ischemia injury and chemical structure of three borneols. (A) Levorotatory borneol (endo-(1 S)-1,7,7-trimethyl-bicyclo [2.2.1] heptan-2-ol, (–)-borneol), which is extracted from fresh leaves of Blumea balsamifera (L.) DC. (B) Dextrorotatory borneol (endo-(1R)-1,7,7-trimethyl-bicyclo [2.2.1] heptan-2-ol, (+)-borneol), which is extracted from fresh branches and leaves of Cinnamomum camphora (L.) Presl. (C) Synthetic borneol (DL-borneol) is an optically inactive (±) borneol that is mainly a mixture of (±) borneol and is obtained via the chemical transformation of camphor and turpentine oil.