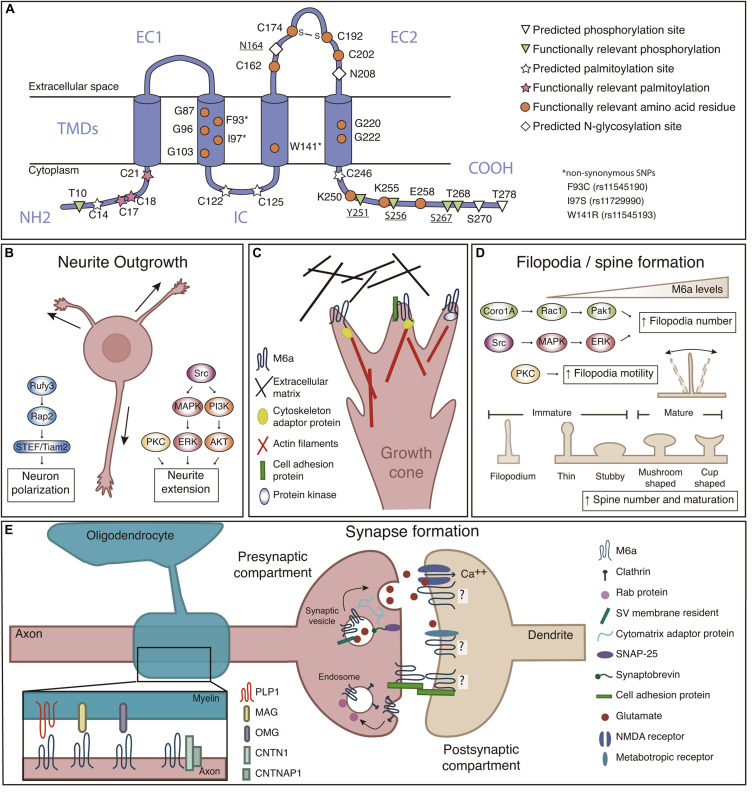
FIGURE 1.
(A) M6a structural features. M6a’s topological computational model predicts four transmembrane domains (TMDs), two extracellular loops (a small one—EC1- and a large one—EC2-), an intracellular loop (IC), and N- and C-terminal ends facing the cell cytoplasm (NH2 and COOH). Predicted sites for palmitoylation (C14, C17, C18, C21, C122, C125, and C246), N-glycosylation (N164 and N208), and phosphorylation (T10, Y251, S256, S267, T268, S270, and T278) are highlighted; bold underlined sites indicate post-translational modifications which are experimentally validated. Orange circles represent amino acid residues relevant for M6a function. (B) M6a promotes neurite outgrowth and neuronal polarization, which involves the activation of Src/MAPK/ERK, PKC and PI3K/AKT, and Rufy3-Rap2-STEF/Tiam2 signaling pathways, respectively. (C) We hypothesize that M6a function depends on its association with partner proteins in specific membrane microdomains. These associations, induce auto/phosphorylation in specific C-terminal residues facing the cytoplasm, and finally promote neuronal plasticity (Fuchsova et al., 2009; Scorticati et al., 2011; Formoso et al., 2015a,b; Garcia et al., 2017; Honda et al., 2017; Aparicio et al., 2020). For example, M6a can associate with extracellular matrix proteins such as brevican and tenascin C through its extracellular loops in trans and/or with cell adhesion proteins (NCAM and NPTN) through its extracellular domains in cis, triggering its phosphorylation and therefore the recruitment of adapter proteins (such as Rap) and the activation of protein kinases that finally promote the reorganization of the cytoskeleton. (D) M6a promotes filopodia formation and motility through a mechanism that involves the activation of the Coro1A/Rac1/Pak1 and Src/MAPK/ERK, and PKC pathways, respectively. M6a also participates in spine formation and maturation of dendritic protrusions. (E) Left: Recently proposed interactions between M6a and myelin sheath proteins (PLP1, MAG, and OMG), and myelinated-axon proteins CNTN1 and CNTNAP1. Right: M6a is located at the presynaptic compartment plasma membrane, synaptic vesicle—SV—and endosomal membranes. M6a also interacts with postsynaptic glutamate receptors (like NMDA-R1 and GRM1) but its localization to the postsynaptic compartment was not experimentally confirmed yet. M6a, glycoprotein M6a; Src, Src kinase; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; PKC, protein kinase C; PI3K, phosphatidylinositide 3-kinase; AKT, serine/threonine-protein kinase; Rap2, Ras-related protein-2; Rufy3, Rufy 3 protein; STEF/Tiam2, T-lymphoma invasion and metastasis-inducing protein 2; NCAM, Neural cell adhesion molecule; NPTN, neuroplastin; Coro1A, coronin-1A; Rac1, Ras-related C3 botulinum toxin substrate 1; Pak1, p21-activated kinase 1; PLP1, proteolipid protein 1; MAG, myelin-associated glycoprotein; OMG, oligodendrocyte-myelin glycoprotein; CNTN1, contactin-1; CNTNAP1, contactin-associated protein 1; NMDA-R1, N-methyl-D-aspartate receptor 1; GRM1, metabotropic glutamate receptor 1; SNAP25, Synaptosomal-Associated Protein, 25 kDa.