Abstract
Background
Lung cancer risk prediction models do not routinely incorporate imaging metrics available on low-dose CT (LDCT) imaging of the chest ordered for lung cancer screening.
Research Question
What is the association between quantitative emphysema measured on LDCT imaging and lung cancer incidence and mortality, all-cause mortality, and airflow obstruction in individuals who currently or formerly smoked and are undergoing lung cancer screening?
Study Design and Methods
In 7,262 participants in the CT arm of the National Lung Screening Trial, percent low attenuation area (%LAA) was defined as the percentage of lung volume with voxels less than –950 Hounsfield units on the baseline examination. Multivariable Cox proportional hazards models, adjusting for competing risks where appropriate, were built to test for association between %LAA and lung cancer incidence, lung cancer mortality, and all-cause mortality with censoring at 6 years. In addition, multivariable logistic regression models were built to test the cross-sectional association between %LAA and airflow obstruction on spirometry, which was available in 2,700 participants.
Results
The median %LAA was 0.8% (interquartile range, 0.2%-2.7%). Every 1% increase in %LAA was independently associated with higher hazards of lung cancer incidence (hazard ratio [HR], 1.02; 95% CI, 1.01-1.03; P = .004), lung cancer mortality (HR, 1.02; 95% CI, 1.00-1.05; P = .045), and all-cause mortality (HR, 1.01; 95% CI, 1.00-1.03; P = .042). Among participants with spirometry, 892 had airflow obstruction. The likelihood of airflow obstruction increased with every 1% increase in %LAA (odds ratio, 1.07; 95% CI, 1.06-1.09; P < .001). A %LAA cutoff of 1% had the best discriminative accuracy for airflow obstruction in participants aged > 65 years.
Interpretation
Quantitative emphysema measured on LDCT imaging of the chest can be leveraged to improve lung cancer risk prediction and help diagnose COPD in individuals who currently or formerly smoked and are undergoing lung cancer screening.
Key Words: chest imaging, COPD, emphysema, lung cancer
Abbreviations: AUC, area under the curve; HR, hazard ratio; LDCT, low-dose CT; %LAA, percent low attenuation area
FOR EDITORIAL COMMENT, SEE PAGE 1699
The National Lung Screening Trial (NLST) showed that annual screening with low-dose CT (LDCT) imaging of the chest decreases death from lung cancer in high-risk current and former smokers.1 Beyond lung cancer screening, LDCT imaging can reveal clinically important intrathoracic abnormalities such as emphysema, airway thickening, bronchiectasis, pleural disease, and coronary artery calcium.2,3 These findings have important implications for the care and prognosis of screened patients but are not yet routinely incorporated in risk prediction models. Quantitative emphysema is an attractive candidate for inclusion in such models given its automated and time-efficient measurement by densitometry methods.4 However, previous studies examining the association between quantitative emphysema and lung cancer incidence and mortality have produced mixed results.5, 6, 7, 8, 9, 10, 11 Notably, these studies were heterogeneous regarding CT types (low-dose vs high-resolution vs cardiac), filter applications for noise reduction, density cutoffs for emphysema definition, and pretest lung cancer risk of participants.
In addition to lung cancer screening, LDCT imaging of the chest represents an opportunity to identify current or former smokers with undiagnosed COPD. This is particularly important because COPD remains substantially underdiagnosed at a population level.12,13 After adjustments for BMI, smoking pack-years, and current smoking status, quantitative emphysema detected on LDCT imaging has been found to be independently associated with COPD.14 Nonetheless, how this association varies with age and sex remains to be determined.
In the current analysis of NLST data, the goal was to define the incremental predictive value of quantitative emphysema measured on noise-filtered LDCT imaging using a cutoff of –950 Hounsfield units for lung cancer incidence, lung cancer mortality, all-cause mortality, and airflow obstruction prevalence within validated risk prediction models.
Patients and Methods
Participants and Measurements
Details of the NLST design have been previously described.15 Briefly, the NLST enrolled individuals between 55 and 74 years of age, with at least a 30 pack-year smoking history, who were either currently smoking or have quit within the past 15 years. Participants were randomized to three annual lung cancer screenings with either LDCT imaging or a single-view chest radiograph. The primary outcome was death from lung cancer. In this analysis, 7,262 participants from the CT arm were included who met desired imaging parameters and had no missing clinical data or follow-up (e-Fig 1).
Data on demographic characteristics, smoking history, self-reported COPD, and personal and family history of cancer were collected at study enrollment. The NLST LDCT acquisition protocol has been previously reported.15 Emphysema was quantified on the baseline screening CT scan as the percent low attenuation area (%LAA), defined as the percentage of lung volume with voxel density less than –950 Hounsfield units, using automated densitometry software (Imbio LLC). Prior to emphysema quantification, a 3 × 3 × 3 median filter was applied to reduce image noise to match that found in standard-dose scans.16 A subset of participants also underwent pre-bronchodilator spirometry at the baseline visit using a SpiroPro spirometer (eResearch Technology GmbH).
The study protocol was approved by the institutional review boards of all study centers. All participants gave written informed consent.
Outcomes
Outcomes of interest in the current analysis were lung cancer incidence and mortality, all-cause mortality, and presence of airflow obstruction on spirometry. In the NLST protocol, a lung cancer diagnosis was confirmed on pathology reports following diagnostic procedures performed for further evaluation of a positive screen, and the vital status of participants was assessed through questionnaires and review of the National Death Index registry, with an end point verification team determining the cause of death. A ratio of FEV1 over the FVC of < 70% on spirometry was used to define airflow obstruction.
Statistical Analyses
Baseline characteristics are presented for all participants and according to %LAA subgroups (%LAA ≤ 1%; 1% < %LAA ≤ 5%; %LAA > 5%) as mean and SD for continuous variables, and as count and proportion for categorical variables.
Multivariable Cox regression models were constructed to test the association between %LAA and lung cancer incidence, lung cancer mortality, and all-cause mortality. Models were individually adjusted for variables relevant to each outcome based on previous NLST analyses by Tammemägi et al17 for lung cancer incidence and by Kovalchik et al18 for lung cancer mortality. We also built parsimonious models adjusted only for age and sex, variables that are routinely available as part of Digital Imaging and Communications in Medicine metadata and that can be incorporated into automated image analysis. To account for dependent censoring from competing causes of death in modeling lung cancer incidence and mortality, we used an inverse probability weighting approach applied to Cox models based on age, sex, race, ethnicity, highest level of education attained, BMI, current smoking status, smoking intensity, smoking duration, time since smoking cessation, self-reported COPD, personal history of cancer, family history of lung cancer, and %LAA. A similar approach was used to generate Kaplan-Meier curves for lung cancer incidence according to %LAA severity groups (%LAA ≤ 1%; 1% < %LAA ≤ 5%; %LAA > 5%). The proportional hazard assumption was checked and met in all full and parsimonious Cox regression models included in this analysis. In all models, participants were censored at 6 years from study entry. For lung cancer incidence, any death without cancer incidence was dependent censoring; loss to follow-up without cancer incidence or cancer-free status at year 6 was independent censoring. For lung cancer mortality, any death not caused by lung cancer was dependent censoring; loss to follow-up or alive vital status at year 6 was independent censoring. For all-cause mortality, loss to follow-up or alive vital status at year 6 was independent censoring.
Logistic regression models adjusted for age and sex were also constructed to test the cross-sectional association between %LAA and airflow obstruction on spirometry. We then calculated the sensitivity, specificity, positive predictive value, negative predictive value, and area under the curve (AUC) to determine the predictive ability of a %LAA cut off of 1% for airflow obstruction in subgroups stratified according to age (< 65 years vs ≥ 65 years) and sex (male vs female).
All analyses were performed in R software version 3.4.1 (R Foundation for Statistical Computing). The following R packages were used: “survival,” “ipw,” and “pROC.” A P value < .05 was considered statistically significant.
Results
Participant Characteristics
The median %LAA was 0.8% (interquartile range, 0.2% to 2.7%). Among all 7,262 participants, 3,965 (54.6%) had %LAA ≤ 1%, 2,204 (30.3%) had %LAA between 1% and 5%, and 1,093 (15.1%) had %LAA > 5%. Mean age of all participants was 61.5 years and increased with increasing %LAA category. The respective proportions of female subjects and current smokers were 41.3% and 48.8%, and decreased with increasing %LAA category. Participants smoked a mean of 28.1 cigarettes per day for a mean duration of 39.9 years. For former smokers, mean time since smoking cessation was 3.8 years. Complete clinical and demographic characteristics of participants are listed in Table 1.
Table 1.
Baseline Characteristics of Participants
| Characteristic | All (N = 7,262) | %LAA ≤ 1% (n = 3,965) | 1% < %LAA ≤ 5% (n = 2,204) | %LAA > 5% (n = 1,093) | P Valuea |
|---|---|---|---|---|---|
| Age, y | 61.5 ± 5.0 | 60.9 ± 4.8 | 61.8 ± 5.1 | 63.2 ± 5.4 | < .001 |
| Female | 2,997 (41.3%) | 1,944 (49.0%) | 749 (34.0%) | 304 (27.8%) | < .001 |
| Race and ethnicity | < .001 | ||||
| Non-Hispanic White | 6,624 (91.2%) | 3,561 (89.8%) | 2,034 (92.3%) | 1,029 (94.1%) | |
| Non-Hispanic Black | 360 (5.0%) | 251 (6.3%) | 85 (3.9%) | 24 (2.2%) | |
| Hispanic or other | 278 (3.8%) | 153 (3.9%) | 85 (3.9%) | 40 (3.7%) | |
| Highest level of school attained | < .001 | ||||
| Less than high school | 430 (5.9%) | 270 (6.8%) | 114 (5.2%) | 46 (4.2%) | |
| High school | 1,586 (21.8%) | 872 (22.0%) | 451 (20.5%) | 263 (24.1%) | |
| Some college, no degree | 925 (12.7%) | 515 (13.0%) | 286 (13.0%) | 124 (11.3%) | |
| Associate/technical degree | 1,698 (23.4%) | 978 (24.7%) | 482 (21.9%) | 238 (21.8%) | |
| Bachelor’s degree | 1,364 (18.8%) | 704 (17.8%) | 434 (19.7%) | 226 (20.7%) | |
| Graduate degree | 1,259 (17.3%) | 626 (15.8%) | 437 (19.8%) | 196 (17.9%) | |
| BMI, kg/m2 | 28.0 ± 5.0 | 28.9 ± 5.3 | 27.4 ± 4.7 | 25.8 ± 4.0 | < .001 |
| Individuals who currently smoke | 3,544 (48.8%) | 2,154 (54.3%) | 992 (45.0%) | 398 (36.4%) | < .001 |
| Smoking intensity (cigarettes/d) | 28.1 ± 10.9 | 28.1 ± 11.0 | 27.6 ± 10.6 | 29.2 ± 11.0 | < .001 |
| Smoking duration, y | 39.9 ± 7.4 | 39.6 ± 7.2 | 40.0 ± 7.5 | 41.2 ± 7.7 | < .001 |
| Time since smoking cessation, y | 3.8 ± 5.1 | 3.4 ± 4.9 | 4.1 ± 5.2 | 4.6 ± 5.1 | < .001 |
| Self-reported COPD | 366 (5.0%) | 118 (3.0%) | 95 (4.3%) | 153 (14.0%) | < .001 |
| Personal history of cancer | 283 (3.9%) | 162 (4.1%) | 91 (4.1%) | 30 (2.7%) | .10 |
| Family history of lung cancer | 1,623 (22.3%) | 910 (23.0%) | 484 (22.0%) | 229 (21.0%) | .32 |
Data are presented as means ± SD for continuous variables and counts (proportions) for categorical variables. %LAA = percent low attenuation area (defined as the percentage of lung volume with voxels less than –950 Hounsfield units on CT imaging of the chest).
P value comparing the three %LAA groups using analysis of variance for continuous variables and the χ2 test for categorical variables.
Emphysema and Lung Cancer Incidence
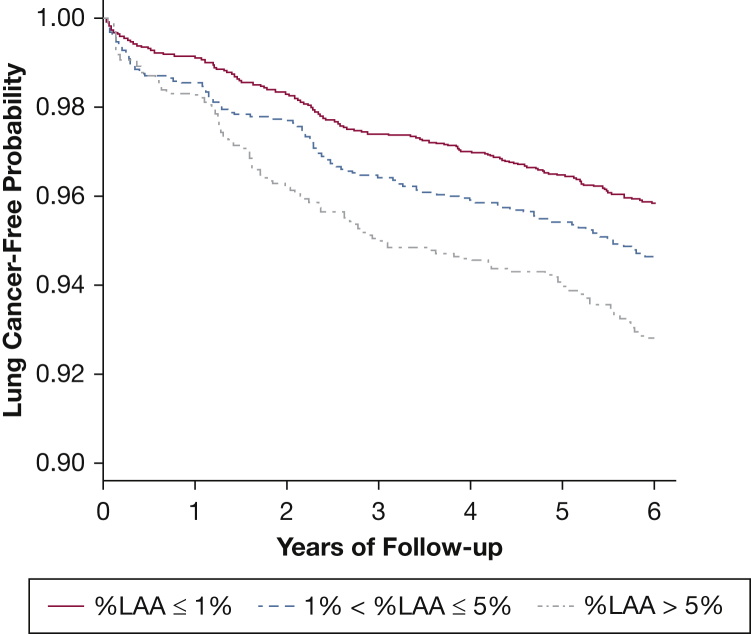
A total of 353 participants developed lung cancer by 6 years of follow-up. Participants with higher %LAA had a higher likelihood of lung cancer incidence as shown in Figure 1 (pairwise comparisons by %LAA severity groups: P = .04 for %LAA 1%-5% vs %LAA ≤ 1%; P = .03 for %LAA > 5% vs %LAA 1%-5%; P < .001 for %LAA > 5% vs %LAA ≤ 1%). In multivariable analyses, each 1% increase in %LAA was associated with an increased hazard of lung cancer incidence (hazard ratio [HR], 1.02 [95% CI, 1.01-1.03; P = 0.004]; C statistic for model, 0.71 [95% CI, 0.68-0.74]) (Table 2). Other variables independently associated with lung cancer incidence included higher age, lower BMI, greater smoking intensity and duration, self-reported COPD, and a family history of lung cancer. In a parsimonious model including only age and sex as covariates, %LAA remained significantly associated with lung cancer incidence (HR, 1.02 per 1% increase [95% CI, 1.01-1.04; P < .001]; C statistic for model, 0.64 [95% CI, 0.61-0.67]).
Figure 1.
Kaplan-Meier curves showing inverse-weighted time-to-event probabilities for lung cancer incidence according to %LAA groups. %LAA = percent low attenuation area.
Table 2.
Multivariable Cox Regression Model of Lung Cancer Incidence
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age (per 1-y increase) | 1.04 (1.00-1.08) | .03 |
| Race and ethnicity | ||
| Non-Hispanic White | 1.00 [reference group] | |
| Non-Hispanic Black | 0.99 (0.59-1.65) | .96 |
| Hispanic or other | 0.43 (0.19-0.96) | .04 |
| Education (per 1-level increase) | 0.94 (0.88-1.01) | .08 |
| BMI (per 1-kg/m2 increase) | 0.97 (0.95-0.99) | .02 |
| Smoking status (current vs former) | 1.04 (0.77-1.40) | .81 |
| Smoking intensity (per 1-cigarette/d increase) | 1.02 (1.01-1.03) | < .001 |
| Smoking duration (per 1-y increase) | 1.06 (1.03-1.09) | < .001 |
| Time since smoking cessation (per 1-y increase) | 0.97 (0.93-1.01) | .20 |
| Self-reported COPD (yes vs no) | 1.45 (1.00-2.10) | .048 |
| Personal history of cancer (yes vs no) | 0.98 (0.57-1.67) | .94 |
| Family history of lung cancer (yes vs no) | 1.39 (1.10-1.76) | .006 |
| %LAA (per 1% increase) | 1.02 (1.01-1.03) | .004 |
%LAA = percent low attenuation area (defined as the percentage of lung volume with voxels less than –950 Hounsfield units on CT imaging of the chest).
Emphysema and Lung Cancer Mortality
A total of 99 participants died of lung cancer by 6 years of follow-up. Each 1% increase in %LAA was independently associated with an increased hazard of lung cancer death (HR, 1.02 [95% CI, 1.00-1.05; P = .045]; C statistic for model, 0.73 [95% CI, 0.67-0.79]) (e-Table 1). Other factors associated with lung cancer death included older age, greater smoking history, shorter time since smoking cessation, and family history of lung cancer. In a parsimonious model including only age and sex, higher %LAA was associated with lung cancer mortality, but this association was not statistically significant (HR, 1.02 per 1% increase [95% CI, 1.00-1.04; P = .08]; C statistic, 0.63 [95% CI, 0.58-0.69]).
Emphysema and All-Cause Mortality
A total of 422 participants died of any cause, including lung cancer, during 6 years of follow-up. Each 1% increase in %LAA was independently associated with higher all-cause mortality (HR, 1.01 [95% CI, 1.00-1.03; P = .042]; C statistic for model, 0.68 [95% CI, 0.65-0.71]) (e-Table 2). Other factors associated with death from any cause were older age, male sex, non-Hispanic Black race and ethnicity, greater smoking history, and shorter time since smoking cessation. In a parsimonious model including only age and sex, higher %LAA was associated with all-cause mortality, but this association was not statistically significant (HR, 1.01 per 1% increase [95% CI, 1.00-1.02; P = .10]; C statistic for model, 0.64 [95% CI, 0.61-0.67]).
Emphysema and Airflow Obstruction Prevalence
Among the 7,262 participants included in this analysis, 2,700 (37.2%) had spirometry performed. Of these, 892 (33.0%) had chronic airflow obstruction. Male subjects had a higher prevalence of airflow obstruction than female subjects (36.9% vs 28.6%; P < .001). Within each sex, individuals aged ≥ 65 years had a higher prevalence of airflow obstruction compared with those aged < 65 years: 43.8% vs 33.9% for male subjects (P < .001) and 36.8% vs 25.6% for female subjects (P < .001). The medians and interquartile ranges for %LAA were 2.0% (0.4%-6.4%) in participants with airflow obstruction and 0.3% (0.1%-1.0%) in those without airflow obstruction. Histograms of %LAA distribution in participants with and without airflow obstruction are shown in e-Figure 2. Many smokers with airflow obstruction had mild emphysema, whereas some smokers without airflow obstruction had a significant burden of emphysema.
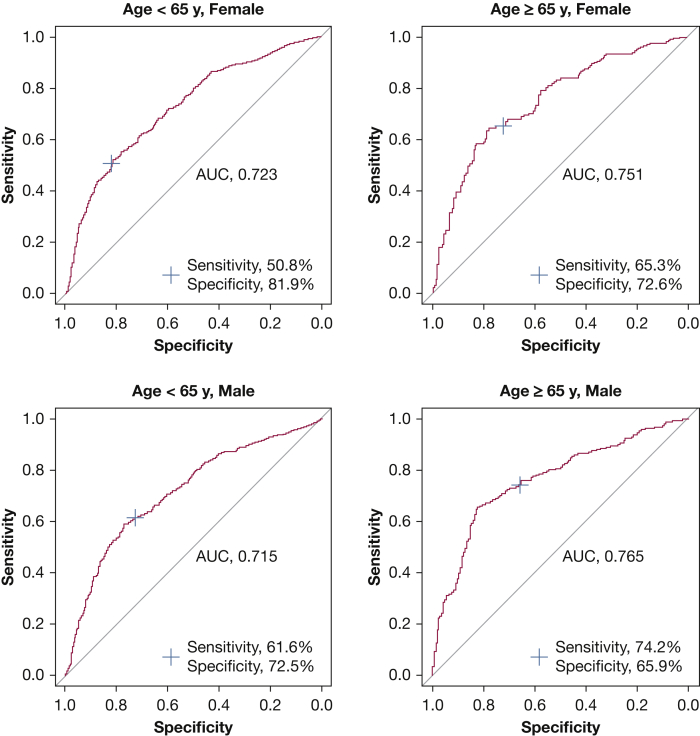
In a logistic regression model adjusted for age and sex, each 1% increase in %LAA was strongly associated with airflow obstruction (OR, 1.07; 95% CI, 1.06-1.09; P < .001). To further characterize this cross-sectional association between airflow obstruction and quantitative emphysema on LDCT imaging, we studied the accuracy of a %LAA cut off of 1% for the presence of airflow obstruction in different age and sex subgroups. This practical cutoff was selected because the optimal %LAA cut off for distinguishing between those with and without airflow obstruction was 1.27% after maximization of the Youden index (sensitivity + specificity, – 1). %LAA > 1% best predicted the presence of airflow obstruction in male subjects aged ≥ 65 years (sensitivity, 74%; specificity, 66%; AUC, 0.77), followed by female subjects aged ≥ 65 years (sensitivity, 65%; specificity, 73%; AUC, 0.75) (Fig 2). The sensitivities, specificities, positive predictive values, negative predictive values, and their 95% CIs according to subgroups of participants are listed in e-Table 3. The highest specificity of %LAA > 1% for the presence of airflow obstruction was in female subjects aged < 65 years.
Figure 2.
Receiver-operating characteristic curves showing the discriminative ability of % low attenuation area > 1% for airflow obstruction in different age and sex subgroups. AUC = area under the curve.
In this group of participants with available spirometry, %LAA did not achieve statistical significance for predicting lung cancer incidence and mortality due to loss of power from reduced sample size but retained effect sizes comparable in magnitude and direction to the entire cohort (e-Table 4). In this same group, airflow obstruction was associated with all outcomes but achieved statistical significance for lung cancer mortality only (HR, 1.77; 95% CI, 1.03-3.05; P = .04).
Discussion
In this analysis of NLST data, we show that quantitative emphysema measured on LDCT imaging is independently associated with lung cancer incidence, lung cancer mortality, and all-cause mortality at 6 years of follow-up in current or former smokers undergoing lung cancer screening. We also show that quantitative emphysema on LDCT imaging is associated with higher odds of airflow obstruction and that a %LAA cut off of 1% can identify airflow obstruction with good accuracy in individuals aged > 65 years.
Although tobacco exposure is the leading cause of both emphysema and lung cancer, emphysema is an established risk factor for lung cancer even after accounting for smoking history.19,20 Persistent inflammation in the lung parenchyma has been proposed as a mechanistic driver of this link between emphysema and lung cancer.21 In their constant attempts to repopulate injured areas of the lung in response to chronic damage induced by cigarette smoking, bronchoalveolar stem cells may in time start to proliferate uncontrollably, thereby leading to tumorigenesis.22 Not only does emphysema provide a milieu conducive to malignancy, but it also promotes tumor growth and aggressiveness. Murakami et al23 showed that stage I non-small cell lung cancers arising from pulmonary emphysema are associated with higher tumor microvessel density, proliferative activity and postoperative recurrence rate compared with cancers arising from non-emphysematous lung.
Emphysema detected visually on CT imaging has been associated with lung cancer incidence and mortality following a dose-response relationship based on the grade of visual emphysema (eg, none, trace, mild, moderate, confluent, advanced).11,24,25 However, visual assessment of emphysema severity remains limited by interobserver variability.26, 27, 28 We show that automated quantification of emphysema measured on noise-filtered LDCT imaging using a cut off of –950 Hounsfield units in current or former smokers undergoing lung cancer screening is also associated with lung cancer incidence and mortality. Therefore, the routine use of emphysema quantification can significantly inform lung cancer risk prediction. To illustrate this point, consider two hypothetical patients with identical demographic characteristics, smoking histories, and personal/family histories of malignancy but with quantitative emphysema on LDCT imaging of 1% for patient A and 10% for patient B. Compared with patient A, patient B has a 20% higher risk of developing lung cancer in the next 6 years according to our risk prediction model. By contrast, had emphysema been treated as a binary variable (present vs absent), estimates of lung cancer risk would have been identical for both patients. Each individual who smoked has a pretest probability of lung cancer based on individual risk factors but a potentially different posttest probability based on CT findings such as emphysema severity and nodule characteristics.
The association between %LAA and lung cancer incidence was maintained when adjusted for age and sex only, which suggests that parsimonious lung cancer risk prediction models are valid and feasible. Age, sex, and %LAA can be automatically extracted from a radiology examination to generate a personalized report that could aid both the patient and the clinician in their discussion of lung cancer risk. Our results also suggest that both quantitative emphysema and airflow obstruction are individually important for predicting long-term outcomes, especially lung cancer incidence for %LAA and lung cancer mortality for FEV1/FVC < 0.7. Although a higher extent of emphysema is generally associated with the presence of airflow obstruction, many individuals with FEV1/FVC < 0.7 had a low burden of emphysema in the current cohort, whereas some individuals with FEV1/FVC ≥ 0.7 had significant emphysema. Therefore, both chest CT imaging and spirometry play a role in risk prediction depending on the clinical manifestations of each individual’s underlying lung disease.
Our results have important implications for the optimization of lung cancer screening. The optimal interval between LDCT screenings remains unknown but is likely affected by baseline risk. It is possible that select lower risk screen-eligible patients could safely undergo screening at intervals longer than 1 year. Considering the presence and severity of emphysema on the first LDCT screening may be helpful in refining risk prediction. Although NLST participants with a negative baseline screen had a significantly lower incidence of and mortality from lung cancer over the course of the trial,29 visual emphysema detected on a negative baseline screen was an independent predictor of a subsequent lung cancer diagnosis.30 Specifically, the number needed to screen was substantially lower in participants with visual emphysema compared with those without emphysema at baseline (28 vs 62 at the second screening, and 40 vs 91 at the third screening). Further research is needed to guide how to best exploit all available demographic, clinical, and radiologic data to improve the accuracy of lung cancer risk prediction and to inform decisions regarding screening frequency. Artificial intelligence, which has had a number of successful applications in clinical medicine, may well serve such a purpose.31 In fact, a machine learning approach applied to chest CT scans of individuals who smoked showed promising results for predicting important outcomes such as respiratory events and deaths.32
The finding of a strong correlation between emphysema and airflow obstruction on spirometry also points to an important collateral benefit of lung cancer screening. The detection of emphysema on LDCT imaging of the chest should alert the prescribing physician to the possibility of underlying COPD if not already diagnosed, particularly in patients with chronic respiratory symptoms (eg, shortness of breath, cough, mucus production). Although COPD affects close to 30 million individuals and is the fourth leading cause of death in the United States,33,34 it remains substantially underdiagnosed.13,35 Factors contributing to underdiagnosis include underutilization of spirometry and attribution of respiratory symptoms to older age or physical deconditioning.36 In this analysis, we show that %LAA as low as 1% predicts airflow obstruction with good accuracy, especially in older individuals. Therefore, a finding of %LAA ≥ 1% should, at the very least, prompt clinical providers to obtain spirometry in symptomatic individuals, if not already performed. Beyond emphysema detection, LDCT imaging of the chest provides a valuable opportunity to deliver better comprehensive care to individuals who smoke and undergo lung cancer screening. Examples include performing smoking cessation interventions at the point of screening for individuals who currently smoke37 and instituting cardiovascular disease risk assessments in individuals with coronary artery calcium detected on LDCT imaging.38
Our analysis has several limitations. First, participants who did not meet acceptable CT reconstruction parameters were excluded due to inability to accurately process their scans through automated densitometry. Further research is needed to understand how to overcome these technical barriers and widen the applicability of these analytic methods. Second, in addition to the severity of emphysema, the type of emphysema (eg, centrilobular, panlobular, paraseptal) is clinically important regarding lung cancer risk and COPD morbidity.39, 40, 41 Such information can currently only be obtained from visual assessments of emphysema. Third, a post-bronchodilator FEV1/FVC < 0.7 is required to establish a diagnosis of COPD but we used pre-bronchodilator spirometry in this analysis.42 Although this approach could result in false-positive findings for the diagnosis of COPD, such misclassification is unlikely to have been frequent given the extensive smoking histories of participants enrolled in NLST. Fourth, our analysis did not study CT metrics other than emphysema that can also significantly contribute to airflow obstruction, such as airway wall thickness and bronchiectasis. Fifth, our analysis did not include data on regional quantitative emphysema, which have the potential to further refine risk prediction models for clinically relevant outcomes. For example, upper-lobe-predominant emphysema has been associated with higher lung cancer incidence and greater 5-year progression of emphysema, whereas lower-lobe-predominant emphysema has been associated with more severe airflow obstruction.43,44
Conclusions
Quantitative emphysema measured on LDCT imaging of the chest is a valuable tool to predict long-term outcomes, and it suggests the diagnosis of COPD in symptomatic individuals who currently or previously smoked and are undergoing lung cancer screening. Further investigation is needed to determine how best to operationalize it on a large scale to generate individualized risk predictions for lung cancer and COPD in actual clinical practice.
Acknowledgments
Author contributions: M. K. H. is the guarantor of the content of the manuscript. W. W. L., M. X., S. M., C. R. H., and M. K. H. contributed substantially to the study design and data analysis. All authors contributed to data interpretation and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: W. W. L. reports grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI); nonfinancial support from Pulmonx; and personal fees from Konica Minolta. C. R. H. reports grants from the NIH/NHLBI; and is an employee and stock option holder at Imbio. L. A. K. is an employee and stock option holder at Imbio. C. J. G. has a financial interest in Imbio. D. A. A. serves on the IASLC Smoking Cessation and Tobacco Control Committee and on the American College of Chest Physicians Board of Regents and Lung Cancer Guidelines Executive Committee. J. L. C. reports grants from the NIH/NHLBI, the Department of Veterans Affairs, and the Department of Defense; and personal fees from AstraZeneca and Novartis. F. J. M. reports grants from the NIH/NHLBI; personal fees from Continuing Education, Forest Laboratories, GlaxoSmithKline, Nycomed/Takeda, AstraZeneca, Boehringer Ingelheim, Bellerophon Therapeutics (formerly Ikaria), Genentech, Novartis, Pearl, Roche, Sunovion, Theravance, CME Incite, the Annenberg Center for Health Sciences at Eisenhower, Integritas, InThought, the National Association for Continuing Education, Paradigm Medical Communications, LLC, PeerVoice, UpToDate, Haymarket Communications, the Western Society of Allergy and Immunology, ProterixBio (formerly BioScale), Unity Biotechnology, Concert Pharmaceuticals, Lucid, Methodist Hospital, Columbia University, Prime Healthcare Ltd., WebMD, PeerView Network, the California Society of Allergy and Immunology, Chiesi, and the Puerto Rico Thoracic Society; and advisory board participation for Janssen. M. K. H. reports grants from the NHLBI; personal fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Verona, Merck and Mylan; and research support from Novartis and Sunovion. None declared (M. X., S. M., A. A., M. C. F., C. A. M., E. A. K.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the National Cancer Institute for access to data collected by the National Lung Screening Trial. This work was conducted as part of an approved project (“NLST-125: Screening for COPD with Lung CT Densitometry”). The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the National Cancer Institute.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [Grants R44CA203050, K24HL138188, and T32HL007749].
Supplementary Data
References
- 1.Aberle D.R., Adams A.M., Berg C.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan L., Choi H., Reid M., Khawaja A., Mazzone P.J. Frequency of incidental findings and subsequent evaluation in low-dose computed tomographic scans for lung cancer screening. Ann Am Thorac Soc. 2017;14(9):1450–1456. doi: 10.1513/AnnalsATS.201612-1023OC. [DOI] [PubMed] [Google Scholar]
- 3.Regan E.A., Lowe K.E., Make B.J. Identifying smoking-related disease on lung cancer screening CT scans: increasing the value. Chronic Obstr Pulm Dis. 2019;6(3):233–245. doi: 10.15326/jcopdf.6.3.2018.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labaki W.W., Martinez C.H., Martinez F.J. The role of chest computed tomography in the evaluation and management of the patient with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(11):1372–1379. doi: 10.1164/rccm.201703-0451PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldonado F., Bartholmai B.J., Swensen S.J., Midthun D.E., Decker P.A., Jett J.R. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138(6):1295–1302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 6.Gierada D.S., Guniganti P., Newman B.J. Quantitative CT assessment of emphysema and airways in relation to lung cancer risk. Radiology. 2011;261(3):950–959. doi: 10.1148/radiol.11110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson D.O., Leader J.K., Fuhrman C.R., Reilly J.J., Sciurba F.C., Weissfeld J.L. Quantitative computed tomography analysis, airflow obstruction, and lung cancer in the Pittsburgh lung screening study. J Thorac Oncol. 2011;6(7):1200–1205. doi: 10.1097/JTO.0b013e318219aa93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith B.M., Pinto L., Ezer N., Sverzellati N., Muro S., Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Johannessen A., Skorge T.D., Bottai M. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 10.Oelsner E.C., Carr J.J., Enright P.L. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71(7):624–632. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr L.L., Jacobson S., Lynch D.A. Features of COPD as predictors of lung cancer. Chest. 2018;153(6):1326–1335. doi: 10.1016/j.chest.2018.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruparel M., Quaife S.L., Dickson J.L. Prevalence, symptom burden and under-diagnosis of chronic obstructive pulmonary disease in a lung cancer screening cohort. Ann Am Thorac Soc. 2020;17(7):869–878. doi: 10.1513/AnnalsATS.201911-857OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez C.H., Mannino D.M., Jaimes F.A. Undiagnosed obstructive lung disease in the United States: associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788–1795. doi: 10.1513/AnnalsATS.201506-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mets O.M., Buckens C.F., Zanen P. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306(16):1775–1781. doi: 10.1001/jama.2011.1531. [DOI] [PubMed] [Google Scholar]
- 15.Aberle D.R., Berg C.D., Black W.C. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilts M., Duzenli C. Image filtering for improved dose resolution in CT polymer gel dosimetry. Med Phys. 2004;31(1):39–49. doi: 10.1118/1.1633106. [DOI] [PubMed] [Google Scholar]
- 17.Tammemägi M.C., Katki H.A., Hocking W.G. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalchik S.A., Tammemägi M., Berg C.D. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young R.P., Hopkins R.J., Christmas T., Black P.N., Metcalf P., Gamble G.D. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34(2):380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 20.Denholm R., Schuz J., Straif K. Is previous respiratory disease a risk factor for lung cancer? Am J Respir Crit Care Med. 2014;190(5):549–559. doi: 10.1164/rccm.201402-0338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houghton A.M., Mouded M., Shapiro S.D. Common origins of lung cancer and COPD. Nat Med. 2008;14(10):1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 22.Kim C.F., Jackson E.L., Woolfenden A.E. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Murakami J., Ueda K., Sano F., Hayashi M., Nishimoto A., Hamano K. Pulmonary emphysema and tumor microenvironment in primary lung cancer. J Surg Res. 2016;200(2):690–697. doi: 10.1016/j.jss.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Wilson D.O., Weissfeld J.L., Balkan A. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zulueta J.J., Wisnivesky J.P., Henschke C.I. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–1223. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bankier A.A., De Maertelaer V., Keyzer C., Gevenois P.A. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999;211(3):851–858. doi: 10.1148/radiology.211.3.r99jn05851. [DOI] [PubMed] [Google Scholar]
- 27.Cavigli E., Camiciottoli G., Diciotti S. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol. 2009;19(7):1686–1692. doi: 10.1007/s00330-009-1320-y. [DOI] [PubMed] [Google Scholar]
- 28.Group C.O.C.W., Barr R.G., Berkowitz E.A. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151–159. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patz E.F., Jr., Greco E., Gatsonis C., Pinsky P., Kramer B.S., Aberle D.R. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;17(5):590–599. doi: 10.1016/S1470-2045(15)00621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yong P.C., Sigel K., de-Torres J.P. The effect of radiographic emphysema in assessing lung cancer risk. Thorax. 2019;74(9):858–864. doi: 10.1136/thoraxjnl-2018-212457. [DOI] [PubMed] [Google Scholar]
- 31.Labaki W.W., Han M.K. Artificial intelligence and chest imaging. Will deep learning make us smarter? Am J Respir Crit Care Med. 2018;197(2):148–150. doi: 10.1164/rccm.201709-1879ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez G., Ash S.Y., Vegas-Sanchez-Ferrero G. Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med. 2018;197(2):193–203. doi: 10.1164/rccm.201705-0860OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley C.M., Sciurba F.C. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA. 2019;321(8):786–797. doi: 10.1001/jama.2019.0131. [DOI] [PubMed] [Google Scholar]
- 34.Kochanek K.D., Murphy S., Xu J., Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;(293):1–8. [PubMed] [Google Scholar]
- 35.de-Torres J.P., Wisnivesky J.P., Bastarrika G., Wilson D.O., Celli B.R., Zulueta J.J. The prevalence of obstructive lung disease in a lung cancer screening cohort: analysis of the National Lung Screening Trial-American College of Radiology Image Network Cohort. Ann Am Thorac Soc. 2019;16(5):641–644. doi: 10.1513/AnnalsATS.201811-817RL. [DOI] [PubMed] [Google Scholar]
- 36.Han M.K., Kim M.G., Mardon R. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132(2):403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 37.Cao P., Jeon J., Levy D.T. Potential impact of cessation interventions at the point of lung cancer screening on lung cancer and overall mortality in the United States. J Thorac Oncol. 2020;15(7):1160–1169. doi: 10.1016/j.jtho.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruparel M., Quaife S.L., Dickson J.L. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140–1146. doi: 10.1136/thoraxjnl-2018-212812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez J., Henschke C.I., Yankelevitz D.F. Emphysema phenotypes and lung cancer risk. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0219187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouronte-Roibas C., Fernandez-Villar A., Ruano-Ravina A. Influence of the type of emphysema in the relationship between COPD and lung cancer. Int J Chron Obstruct Pulmon Dis. 2018;13:3563–3570. doi: 10.2147/COPD.S178109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith B.M., Austin J.H., Newell J.D., Jr. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1) doi: 10.1016/j.amjmed.2013.09.020. 94 e97-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Global Initiative for Chronic Obstructive Lung Disease 2020 Report. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
- 43.Bae K., Jeon K.N., Lee S.J. Severity of pulmonary emphysema and lung cancer: analysis using quantitative lobar emphysema scoring. Medicine (Baltimore) 2016;95(48) doi: 10.1097/MD.0000000000005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boueiz A., Chang Y., Cho M.H. Lobar emphysema distribution is associated with 5-year radiological disease progression. Chest. 2018;153(1):65–76. doi: 10.1016/j.chest.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.