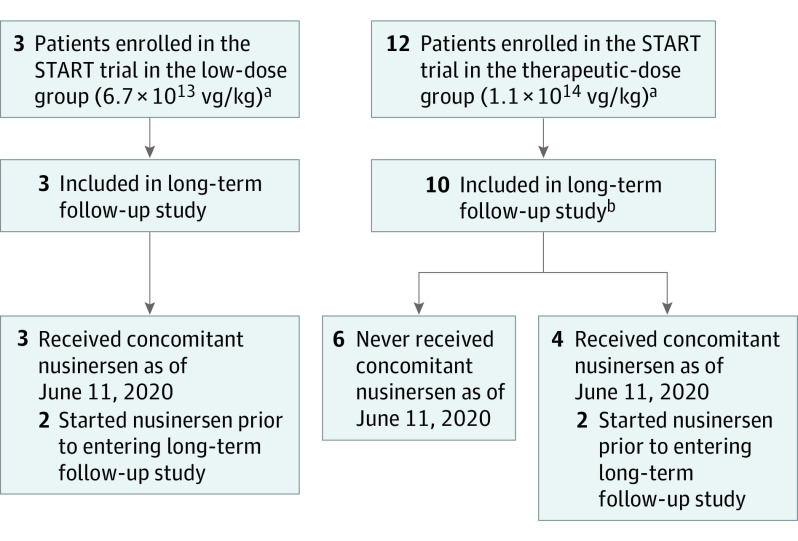
Figure 1. Disposition of Patients in the START LTFU Study.
aNo patient was treated with concomitant nusinersen during the START phase 1 trial.
bTwo patients who did not enroll in the START long-term follow-up study are being followed up at the Nationwide Children’s Hospital Spinal Muscular Atrophy Clinic in Columbus, Ohio. Note that no visits were conducted in the START LTFU clinical trial from December 31, 2019, through June 11, 2020.