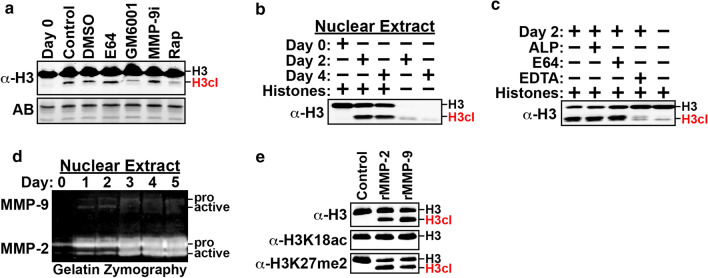
Fig. 2.
MMP-2 is a novel H3NT protease that localizes to the nucleus during myoblast differentiation. a Western analysis to detect the H3cl product in chromatin of C2C12 cells before (Day 0) and after culturing in differentiation media (Day 4) supplemented with DMSO (vehicle) or the inhibitors E64 (cathepsin), GM6001 (MMP), MMP-9i (MMP-9 specific) or rapamycin (myogenic). Amido black staining (AB) of membranes was performed to confirm equivalent loading of chromatin between samples. b In vitro H3 cleavage assay using nuclear soluble extracts purified from C2C12 myoblasts (day 0), differentiating cells (day 2) and myotubes (day 4) incubated alone or with HeLa core histone substrates. Western analysis was performed to detect a cleaved H3 (H3cl) product. (c) In vitro H3 cleavage assay using nuclear soluble extracts from differentiating C2C12 cells (day 2) incubated with selective inhibitors of specific protease families prior to addition of core histone substrates. Inhibitors include a cocktail of aprotinin (serine), leupeptin (serine/cysteine) and pepstatin (aspartyl), E64 (cathepsin) or EDTA (MMP). d Representative gelatin zymography using equivalent amounts of nuclear soluble extracts collected from differentiating C2C12 cells at the indicated time points. The proform of MMP-9 (92 kDa) and MMP-2 (72 kDa) and their cleaved active forms are indicated. e In vitro H3 cleavage assay using core histone substrates in the presence or absence of active recombinant MMP-2 or MMP-9. Western analysis was performed to detect the H3cl product using the indicated antibodies