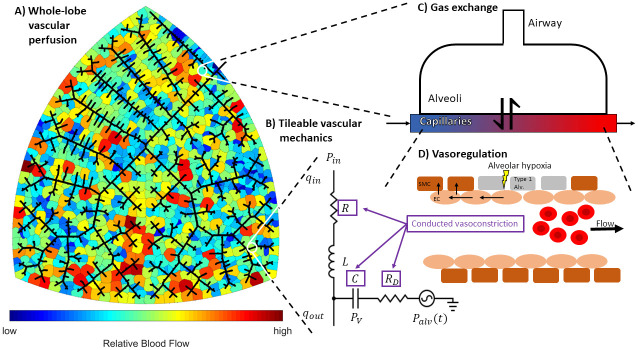
Fig 1. Schematic of our multi-scale multi-physics model of ventilation-perfusion matching.
Block (A) illustrates the whole-lobe vascular network model. Black lines represent blood vessels, and colored regions represent discrete zones of perfusion. The network geometry is agnostically generated by a space-filling algorithm inspired by Wang et al. [33]. Block (B) shows how the mechanics of each vessel segment is represented as an equivalent circuit. Intravascular pressure (Pv) and flow into the vessel (qin) are the state variables; inlet pressure (Pin) and flow out of the vessel (qout) are the initial conditions at boundaries for a given vessel segment; outlet pressure (Pout) is an algebraic constraint; time-varying alveolar pressure (Palv) is a dynamic pressure source; and hydraulic resistance (R), inertance (L), compliance (C), and vessel wall resistance (RD) are anatomical parameters calculated from the geometry of the vessel segment (length and radius) and can be modulated by vasoregulation (purple boxes). Block (C) depicts a representative gas exchange unit. Gases flowing through the capillary tube are exchanged with an alveolar compartment. Block (D) portrays the oxygen-sensitive vasoregulatory mechanism hypoxic pulmonary vasoconstriction (HPV). Hypoxia in the alveolar space induces conducted vasoconstriction. Conducted vascular responses are spatially propagated in a upstream through the endothelium—these responses modulate the values of the anatomical parameters (R, C, and RD) in the arterial network.