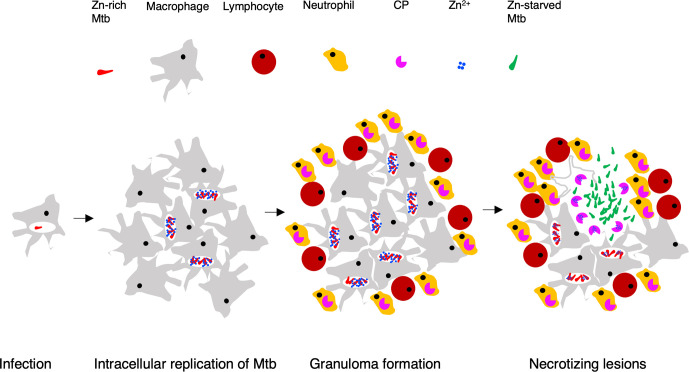
Fig. 5.
A working hypothesis for changing zinc availability to Mtb during progressive TB. Following initial infection, intracellular growth of Mtb is accompanied by enrichment of zinc in the phagosomes [117–119], leading to the expression of C+ribosomes in the pathogen. Infiltrating neutrophils during granuloma formation employ various anti-bacterial mechanisms, including the secretion of zinc-chelating calprotectin (CP) as a component of nutritional immunity [121]. Subsequent lysis of infected macrophages by Mtb-derived factors and Mtb-specific cytotoxic T-cells would cause release of bacilli in extracellular space, where free zinc concentration is expected to be reduced by CP. As a result, Mtb cells will sense zinc starvation and induce C- ribosome expression to conserve intracellular zinc. Induced expression of the C- ribosome is perhaps followed by its hibernation upon further zinc deprivation during exuberant extracellular growth of bacilli.