Figure 2.
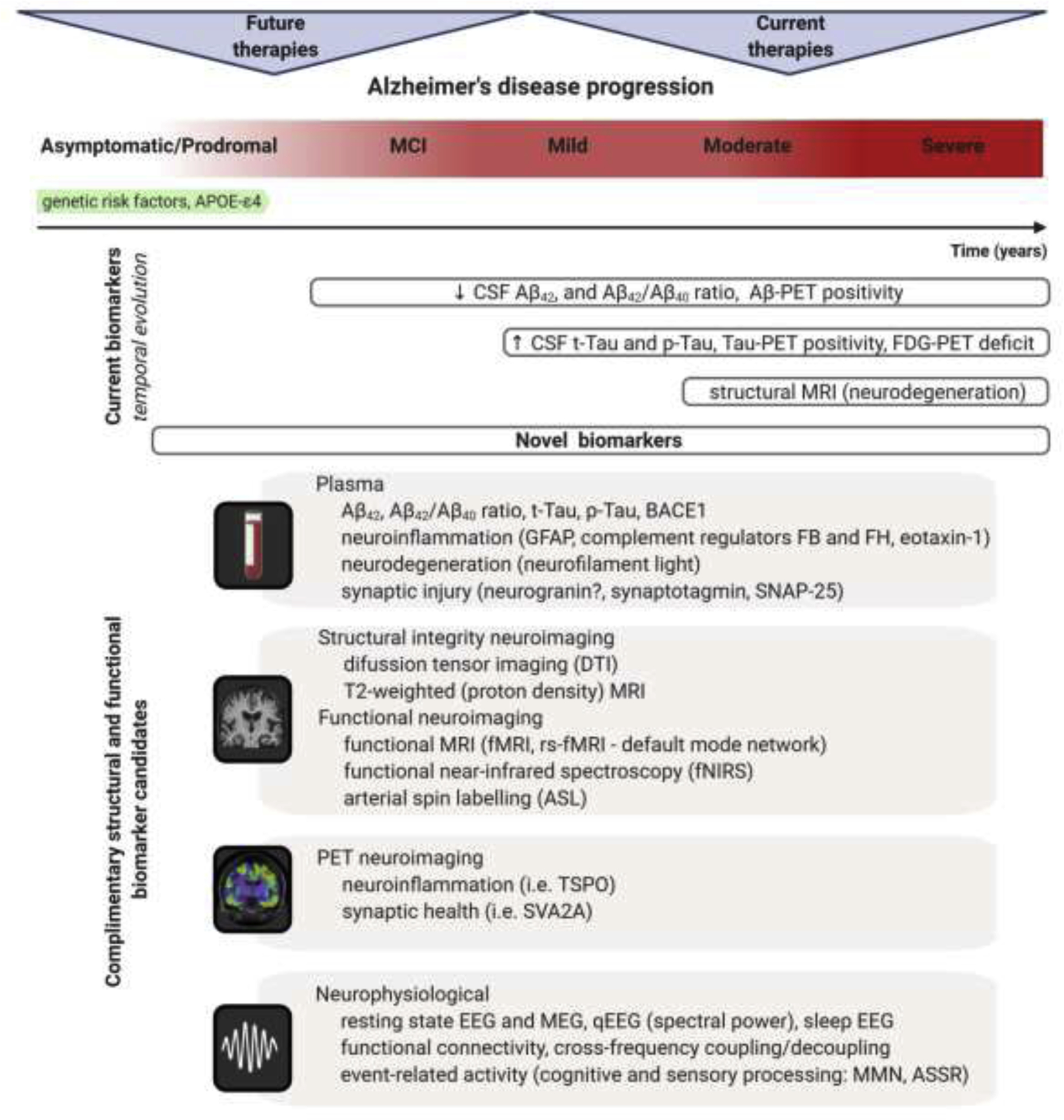
Dynamical changes of biomarkers reflecting core AD pathologies during the course of disease progression. The process of AD begins in the brain with dormant deterioration of neurons and circuits lasting years, probably decades, before the first symptoms emerge. This asymptomatic/prodromal period of the disease represents the desirable window for disease-modifying therapeutic interventions. Currently used biomarker toolbox comprising of cerebrospinal fluid (CSF) and positron emission tomography (PET) measures of Aβ and tau has high diagnostic value indicating AD pathological processes, but cannot reliably index subtle functional changes in the advent of cognitive symptoms or predict treatment response in clinical trials. Additionally, 18F-2fluoro-2-deoxy-D-glucose PET (FDG-PET), measuring neuronal activity through resting state metabolic rate of glucose in the brain, as well as magnetic resonance imaging (MRI) measuring degree of neurodegeneration are nonspecific markers and may indicate several etiologies of brain damage. Improved analytical techniques facilitate development of novel biomarkers with potential for refined and/or less invasive measuring of AD associated morphological and functional alterations through diverse transition states of the disease. Plasma Aβ and tau profiles, and set of specific markers for neuroinflammation, neurodegeneration, and synaptic injury have emerged as clinically valid measures for early diagnosis and prediction of disease progression (Hampel et al., 2020; Mila-Aloma et al., 2019; Morgan et al., 2019). Advanced neuroimaging methods demonstrate high degree of sensitivity and specificity for detection of initial pathologic and functional changes which are important for staging progression and for selecting patients for clinical trials during the prodromal phase of AD (Marquez and Yassa, 2019). Certain neurophysiological signals and applied innovative approaches for analyses are proven to be valid proxies for subtle abnormalities at neural network level and for assessment of global brain function (Babiloni et al., 2020; Stoiljkovic et al., 2019). Pertinent combination of these novel biomarkers with traditional AD biomarkers can enable more sensitive tracking of early disease-associated alterations and specifically estimate the likelihood of responding to next generation AD treatment. Abbreviations: MCI – mild cognitive impairment; GFAP – glial fibrillary acidic protein; FB – complement factor B; FH – complement factor H; SNAP-25 – synaptosomal-associated protein, 25kDa; rs-fMRI – resting state functional magnetic resonance imaging; TSPO – translocator protein; SVA2A – synaptic vesicle protein 2A; MEG – magnetoencephalography; qEEG – quantitative electroencephalography; MMN – mismatch negativity; ASSR – auditory steady-state response.
