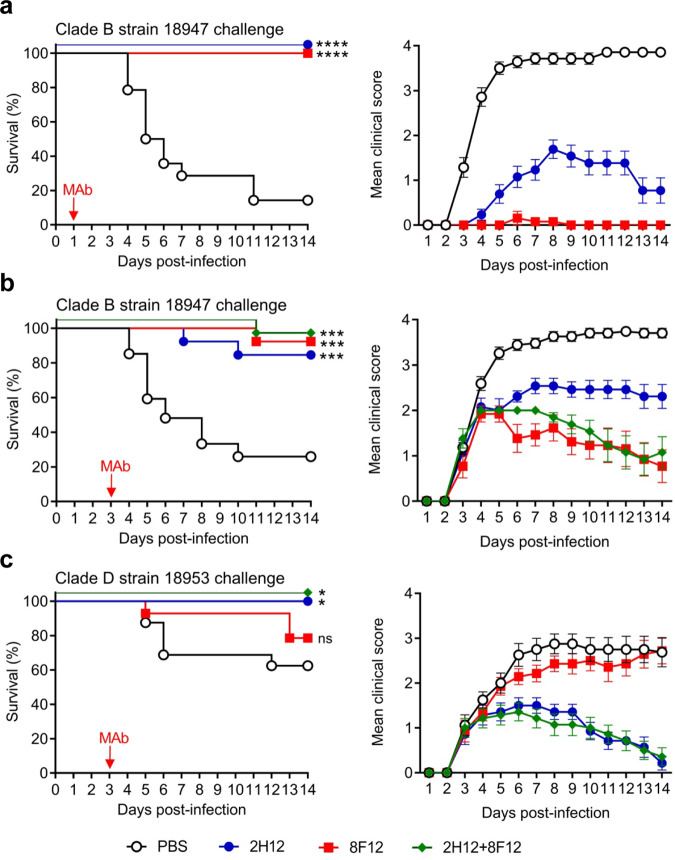
Fig. 2. Therapeutic efficacy of the MAbs against EV-D68 infection in mice.
One-day-old ICR mice (n = 13–27/group) were inoculated intraperitoneally (i.p.) with strain 18947 (a–b) or strain 18953 (c). The suckling mice were i.p. injected with PBS, 10 µg/g of 2H12, 10 µg/g of 8F12, or a mixture of both MAbs (10 μg/g of each MAb) at 1 day post infection (dpi) (a) or 3 dpi (b, c) and were then monitored daily for survival and clinical score. Red arrows indicate the time points of MAb administration. Clinical scores were graded as follows: 0, healthy; 1, lethargy and reduced mobility; 2, limb weakness; 3, limb paralysis; 4, death. Note that to prevent overlap, the overlapping data sets in left panels (survival curves) were nudged by 5 units in the Y direction. Survival rates of antibody-treated mice were compared with the mice in the PBS control group. Statistical significance was determined by Log-rank (Mantel-Cox) test and was indicated as follows: ns., no significant difference (p ≥ 0.05); *, p < 0.05; ***, p < 0.001; ****, p < 0.0001. In panel (a), p value between the 2H12 or 8F12 group and the PBS control group is below 0.0001. In panel (b), p value between the 8F12 or 2H12+8F12 group and the PBS group is 0.0001; p value between the 2H12 group and the PBS group is 0.0007. In panel (c), p value between the 2H12 or 2H12+8F12 group and the PBS group is 0.0121; p value between the 8F12 group and the PBS group is 0.2958. All error bars represent SEM.