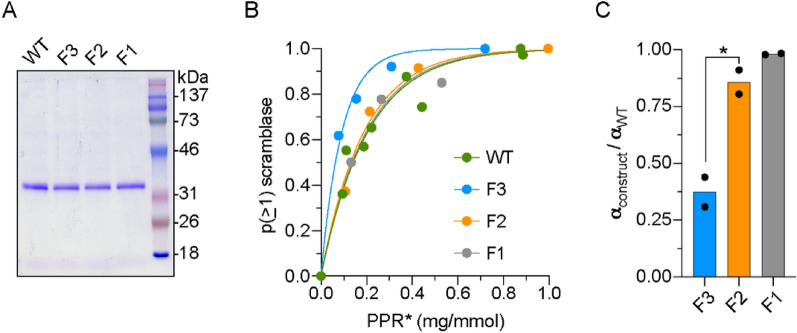
Figure 9.
Reconstitution of WT opsin and TM5 mutants into liposomes. (A) Coomassie-stained SDS-PAGE of purified WT opsin and the F1 (F228A), F2 (F221A, F228A) and F3 (F220C, F221A, F228A) opsin constructs. Molecular weight markers are indicated. (B) Protein dependence of the reconstitution of vesicles with scramblase activity. NBD-PC-containing vesicles were reconstituted with different amounts of the indicated opsin construct and the extent of fluorescence reduction on dithionite treatment was determined by curve-fitting. The data were processed to calculate the probability "p(≥ 1) scramblase" that an individual vesicle in the ensemble possesses a functional scramblase. The graph shows "p(≥ 1) scramblase" versus the corrected protein/phospholipid ratio (PPR*) of the sample (the correction eliminates the contribution of vesicles that are refractory to reconstitution). (C) Ratio of mono-exponential fit constants (α) obtained from analyses of the reconstitution of WT and mutant opsins similar to (and including) the analysis shown in (B). The ratio αconstruct/αWT ~ 1 for F1 and F2 opsins, indicating that these proteins reconstitute similarly to WT opsin as dimers or higher order multimers. However, αF3/αWT ~ 0.4, consistent with reconstitution of F3 as a monomer (αWT = 0.25 ± 0.07, mean ± S.D.). The bar chart is constructed from the results of three independent experiments, each involving 4–5 reconstitutions at different protein/phospholipid ratios (*, p = 0.03, unpaired t-test (two-tail)).