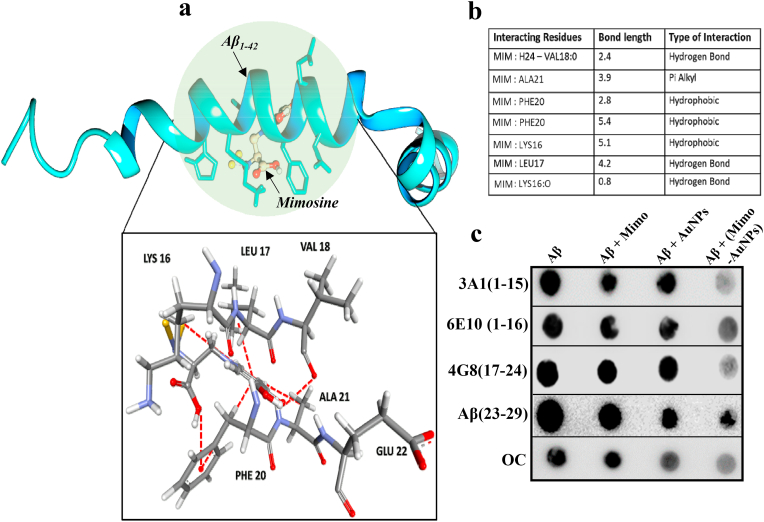
Fig. 4.
Molecular interactions of Mimo-AuNPs with Aβ1-42. (a) Molecular docking data using Auto dock Vina revealing the interaction of Mimo-AuNPs with the monomeric Aβ1-42 representing 7 viable interactions including 3 hydrogen, 3 hydrophobic and 1 pi-alkyl interaction with amino acid residues such as Valine, Alanine, Phenylalanine, Lysine, and Leucine, respectively with an interaction energy of −4.2 kcal mol−1. (b) Table listing all the viable interactions between Mimo-AuNPs and Aβ1-42 PDB ID:1IYT showing its respective bond length in (Å). (c) Epitope mapping of Aβ1-42 conformers using filter-trap assay revealing that Mimo-AuNPs interact with the hydrophobic domain of Aβ1-42.