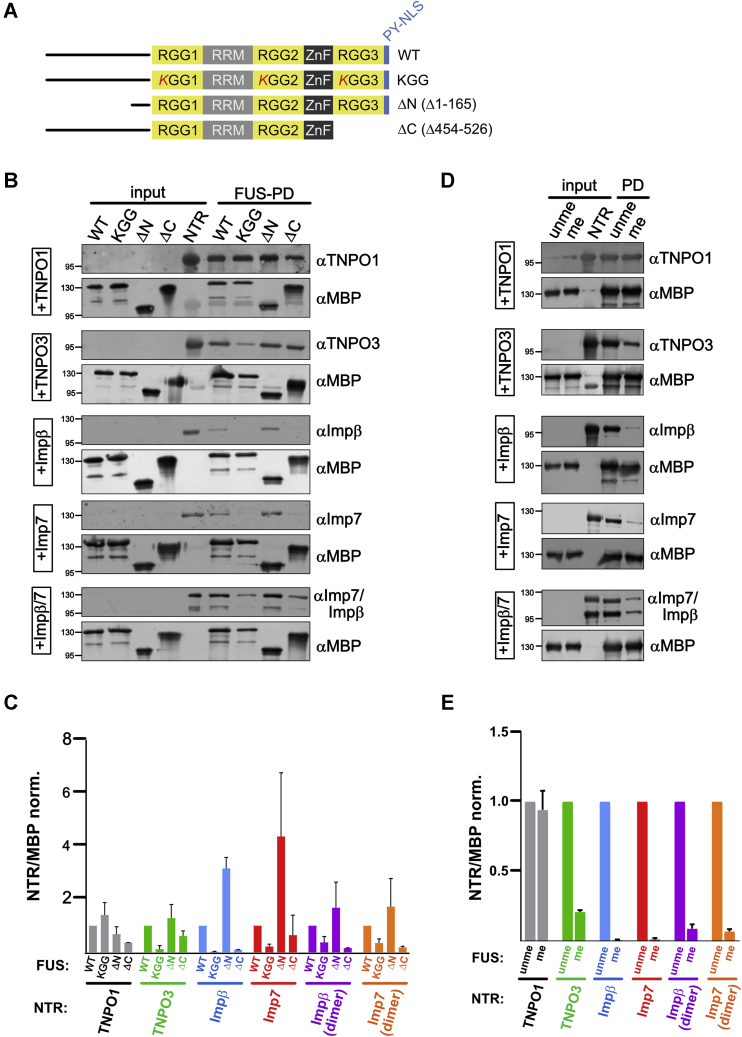
Figure 5.
TNPO3, importin β, importin 7, and importin β/7 bind to RGG motifs in FUS and are affected by arginine methylation.A, scheme illustrating FUS constructs used for pull-down experiments. B, MBP–FUS WT, a FUS mutant with all RG/RGG motifs mutated to KG/KGG (KGG) or FUS deletion mutants lacking either the N-terminal low-complexity domain (aa1-165; ΔN) or the RGG3–PY domain (aa454–526; ΔC) were immobilized on amylose resin and incubated with TNPO1, TNPO3, importin β, importin 7, or importin β/7 as indicated. Binding of importins to the different FUS proteins was analyzed by Western blotting. Successful immobilization of MBP–FUS proteins was verified by MBP Western blot. Input, 15%. C, quantification of the amount of NTR bound to MBP–FUS wt or mutant as the mean of two independent replicates ± SD normalized to TNPO1. D, binding of TNPO1, TNPO3, importin β, importin 7, or importin β/7 to either unmethylated (unme) or methylated (me) MBP–FUS as detected by Western blotting. Immobilization of MBP–FUS proteins was verified by Western blotting. Input, 15%. E, quantification of the amount of NTR bound to unmethylated (unme) or methylated (me) MBP–FUS shown as ratio of NTR/MBP as the mean of three independent replicates ± SEM normalized to unme FUS. NTR, nuclear transport receptor; PY, proline–tyrosine; RGG3, arginine–glycine–rich.