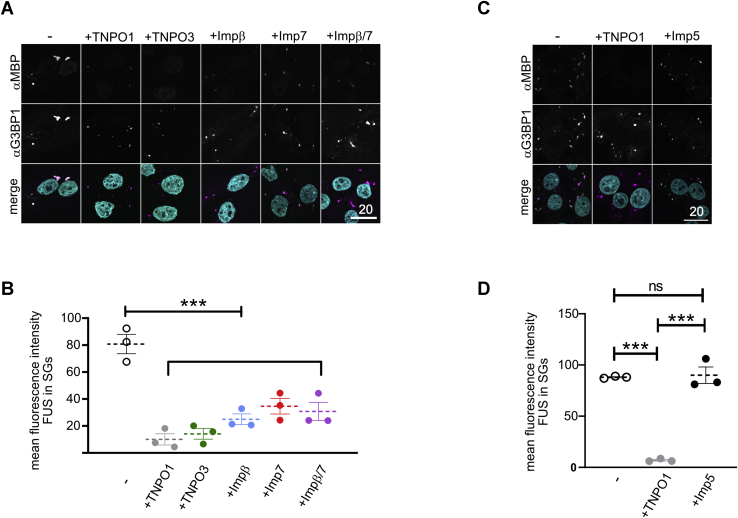
Figure 7.
TNPO3, importin β, importin 7, and importin β/7 suppress stress granule association of FUS.A, association of MBP–FUS to stress granules (SGs) in semipermeabilized cells is suppressed by TNPO1, TNPO3, importin β, importin 7, and importin β/7. SGs and MBP–FUS were visualized by staining for the SG marker protein G3BP1 and the MBP-tag. For clarity, signals were converted to gray values in the individual channels (upper two rows). In the merge (lower row), G3BP1 alone is shown in magenta. White pixels indicate colocalization with MBP–FUS (green). DAPI (turquoise) was included in the merge. The scale bar represents 20 μm. B, quantification of the mean fluorescence intensity of MBP–FUS in SGs for three independent replicates ± SEM, ∗∗∗p < 0.0002 defined by one-way ANOVA with Dunnett's multiple comparison test (≥10 cells; ≥ 32 SGs per condition) in the absence or presence of 5-fold excess of TNPO1, TNPO3, importin β, importin 7, or importin β/7. C, importin 5 cannot suppress association of MBP–FUS to stress granules (SGs) in semipermeabilized cells. SGs and MBP–FUS were visualized by staining for the SG marker protein G3BP1 and the MBP tag as before. The scale bar represents 20 μm. D, quantification of the mean fluorescence intensity of MBP–FUS in SGs for three independent replicates ± SEM, ∗∗∗p < 0.0002 defined by one-way ANOVA with Dunnett's multiple comparison test (≥10 cells; ≥ 30 SGs per condition) in the absence or presence of either TNPO1 or importin 5. G3BP1, Ras GTPase-activating protein-binding 1; SGs, stress granules.