Figure 6.
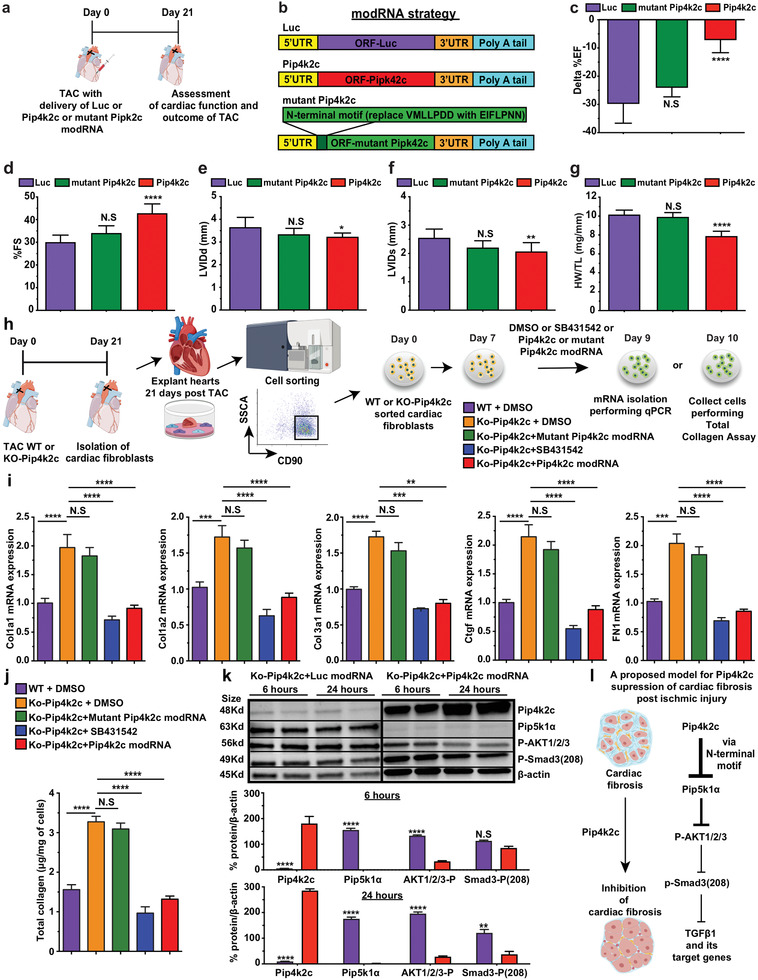
Pip4k2c suppresses the TGFβ1 pathway via its N‐terminal motif (VMLLPDD), which inhibits Pip5k1α, phospho‐AKT 1/2/3, and phospho‐Smad3. a) Experimental plan to evaluate the impact of mutant Pip4k2c (original N‐terminal motif VMLLPDD replaced with EIFLPNN) on cardiac function in the TAC mouse model. b) modRNA strategy for the three modRNA tested. c–f) Echo evaluation of delta % left ventricular ejection fraction (c), fractioning shorting (d), LVIDd (e), and LVIDd (f) 21 days post TAC injury and delivery of Luc, Pip4k2c, or mutant Pip4k2c modRNA (Luc‐, n = 3; Pip4k2c‐ n = 3; mutant Pip4k2c modRNA‐ n = 6). g) Heart weight to tibia length 21 days post TAC injury and delivery of Luc or Pip4k2c modRNA (Luc‐, n = 3; Pip4k2c‐ n = 3; mutant Pip4k2c modRNA‐ n = 6). h) Experimental plan to evaluate Pip4k2c's effect on fibrosis marker expression in cardiac fibroblasts isolated and sorted (for the fibroblastic marker CD90) from WT or KO‐Pip4k2c hearts 21 days post TAC injury and treated with DMSO (control), TbetaR1/ALK5 inhibitor (SB431542), Pip4k2c, or mutant Pip4k2c modRNA i) qPCR analysis of fibrosis markers placed downstream of TGFβ1 following different treatments (n = 3). j) Estimation of total collagen in cells 3 days after different treatments (n = 3). k) Western blot analysis for isolated KO‐Pip4k2c cardiac fibroblasts for Pip4k2c modRNA's effects on the expression of Pip5k1α, phospho‐AKT 1/2/3, and phospho‐Smad3. l) A proposed model for the molecular pathway regulated by Pip4k2c's N‐terminal motif on TGF‐β1 in cardiac fibroblasts. One‐way ANOVA, Tukey's Multiple Comparison Test were used in (c–g) and (i,j). An unpaired two‐tailed t‐test was used for k. ****, P < 0.0001, ***, P < 0.001, **, P < 0.01. *, P < 0.05 N.S., Not Significant.
