Abstract
Introduction:
Extraintestinal manifestations (EIMs) in patients with ulcerative colitis (UC) are common. Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. We evaluated the efficacy of tofacitinib in patients with EIMs, and the impact of tofacitinib on EIMs in patients with UC in the OCTAVE clinical program.
Methods:
Data from two 8-week induction studies (OCTAVE Induction 1 and 2) and a 52-week maintenance study (OCTAVE Sustain) were analyzed. The effect of tofacitinib on efficacy outcomes stratified by EIM status, proportion of predefined prior and active EIMs at baseline, and change from baseline in EIMs were determined at the end of the treatment period (weeks 8 or 52), or at early termination.
Results:
At baseline of OCTAVE Induction 1 and 2, and OCTAVE Sustain, 27.0% and 9.0% of patients had a history of EIMs (prior or active), respectively. Patients treated with tofacitinib 10 mg twice daily (BID) achieved remission and had endoscopic improvement in all studies, irrespective of any history of EIMs. A greater proportion of patients had active peripheral arthritis at baseline of OCTAVE Induction 1 and 2 versus OCTAVE Sustain. In OCTAVE Induction 1 and 2, similar proportions of tofacitinib and placebo-treated patients with active peripheral arthritis experienced either no change (81.3% and 85.7%, respectively) or an improvement (15.6% and 14.3%, respectively). By week 52 of OCTAVE Sustain, improvements in active peripheral arthritis were only observed in tofacitinib-treated patients (16.7% and 33.3% with tofacitinib 5 and 10 mg BID, respectively).
Conclusion:
Any history of EIMs did not influence the efficacy of tofacitinib 10 mg BID for induction or maintenance of UC. The most common active EIM was peripheral arthritis, for which many patients in OCTAVE Induction 1 and 2, and OCTAVE Sustain, reported improvement or no change from baseline with tofacitinib treatment.
Keywords: extraintestinal manifestations, tofacitinib, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition, characterized by mucosal inflammation in the colon.1 In addition to gastrointestinal symptoms, up to one third of patients with UC experience extraintestinal manifestations (EIMs).1 EIMs can have a major impact on patients’ quality of life and increase the morbidity and disability of patients with UC.2,3 The presence of EIMs has previously been reported significantly to increase the risk of poor clinical outcomes, such as lower remission rates.4 The clinical presentation of EIMs is diverse and typically can involve the following four organs: skin (erythema nodosum, pyoderma gangrenosum, psoriasis, aphthous stomatitis); joints (peripheral arthritis, axial arthropathy); hepatobiliary tract (primary sclerosing cholangitis); and eyes (uveitis and episcleritis).5 The relationship between EIMs and UC activity has been reported to vary depending on the type of EIM; some EIMs – such as peripheral arthritis (large joints) – parallel intestinal disease activity, whereas others – such as uveitis – follow an independent course.5–7 EIMs may occur before the onset of intestinal disease,5 and the presence of one EIM increases the likelihood of a patient developing subsequent EIMs.8 Female gender, older age, smoking, longer disease duration, and markers of severe disease [including inflammatory bowel disease (IBD)-related surgery and treatment with tumor necrosis factor inhibitors (TNFis)] are known risk factors for EIMs of arthritis and arthralgia.9
Like UC, the pathogenesis of EIMs involves a combination of genetic, immune response, and environmental factors,1,10 although the mechanism underlying the development of EIMs is not clearly understood; one hypothesis suggests that an immune response may result from shared epitopes between the gastrointestinal mucosa and extraintestinal sites.10 In patients with UC, there is limited evidence regarding the efficacy of specific EIM treatments.11 A systematic review investigating the efficacy and effectiveness of TNFis in the treatment of EIMs reported that TNFis had a clinical benefit on musculoskeletal, cutaneous, and ocular EIMs; however, due to small sample sizes and a limited number of studies, definitive conclusions could not be drawn.11 Although treatment with TNFis can be beneficial for the management of EIMs, approximately a third of patients with UC fail to respond to TNFi induction therapy, and relapse in responders is common, with 23–46% of patients losing response over time.12
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC, rheumatoid arthritis, and psoriatic arthritis.13 The efficacy and safety of tofacitinib has been demonstrated in patients with moderately to severely active UC in three phase III studies [two identical 8-week induction studies (OCTAVE Induction 1 and 2) and a 52-week maintenance study (OCTAVE Sustain)].14 Tofacitinib acts by preferentially inhibiting JAK1 and JAK3 with reduced inhibition for JAK2 and tyrosine kinase 2 which, in turn, inhibits the signal transduction activity by the surface receptors for multiple cytokines.15 The JAK–STAT pathway has been shown to play a role in the pathogenesis of some inflammatory joint and cutaneous conditions,16,17 and has been shown to be upregulated in cutaneous EIMs.18 The objectives of this study were to understand the impact of tofacitinib treatment on the underlying EIM (if present and active at baseline), and to understand how prior or active EIMs influence the response to tofacitinib over time.
Methods
Patients and study design
OCTAVE Induction 1 and 2 (NCT01465763 and NCT01458951) were two identical, 8-week, phase III randomized, double-blind, placebo controlled trials that evaluated the efficacy and safety of tofacitinib as induction therapy for patients with UC.14 Full eligibility criteria have previously been reported in detail.14 Briefly, eligible patients (aged ⩾18 years) were required to have a diagnosis of moderately to severely active UC defined by a total Mayo score of ⩾6, with a rectal bleeding subscore of ⩾1 and an endoscopic subscore of ⩾2. Patients were also required to have failed or be intolerant to conventional therapy for UC [corticosteroids, azathioprine, 6-mercaptopurine, or TNFis (infliximab or adalimumab)]. Only patients who achieved a clinical response at week 8 of OCTAVE Induction 1 and 2 (defined as a decrease from induction study baseline total Mayo score of ⩾3 points and ⩾30%, plus a decrease in rectal bleeding subscore of ⩾1 point or an absolute rectal bleeding subscore of 0 or 1) were eligible to enter the maintenance study, OCTAVE Sustain. OCTAVE Sustain (NCT01458574) was a 52-week, phase III randomized, double-blind, placebo controlled trial that evaluated the efficacy and safety of tofacitinib as maintenance therapy for patients with UC.14 Patients with, or evidence of, suspected liver disease were excluded from the studies.
All three phase III studies were carried out from April 2012 to May 2016. These studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and were approved by the institutional review boards and/or independent ethics committees at each of the investigational centers participating in the studies, or a central institutional review board. All patients provided written informed consent.
Treatment
Patients in OCTAVE Induction 1 and 2 were randomly assigned (4:1) to receive tofacitinib 10 mg twice daily (BID) or placebo for 8 weeks. Patients in OCTAVE Sustain were randomly assigned (1:1:1) to receive tofacitinib 5 mg BID, tofacitinib 10 mg BID, or matched placebo for 52 weeks. Patients were permitted to use concomitant oral corticosteroids (up to 25 mg/day oral prednisone or equivalent, providing the dose was stable within 2 weeks of baseline; tapering of corticosteroids was mandatory during OCTAVE Sustain), 5-aminosalicylate, or sulfasalazine (stable dose within 4 weeks of baseline). Prohibited concomitant medications included: azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, mycophenolate, tacrolimus, interferon, TNFis, and intravenous or rectally administered corticosteroids.
Assessments
A complete medical history, including any history (prior or active) of EIMs (yes/no) was obtained at baseline of OCTAVE Induction 1 and 2, and the status of EIMs was updated at baseline of OCTAVE Sustain. EIMs were selected from a predefined list, specified as being prior (not currently active) or active, and included: peripheral arthritis, oral ulcer/stomatitis, erythema nodosum, thromboembolic disorder, uveitis/iritis, sacroiliitis, pyoderma gangrenosum, myopathy, scleritis/episcleritis, and ankylosing spondylitis. The frequency and proportion of patients with any history of EIMs, and predefined prior (not currently active) and active EIMs, are reported at baseline of OCTAVE Induction 1 and 2, and OCTAVE Sustain. Change from baseline in EIMs was determined at the end of the treatment period (week 8 in OCTAVE Induction 1 and 2; week 52 in OCTAVE Sustain), or at early termination, and was categorized as: no change or new occurrence for patients who never had an EIM at baseline; no change or worsened for patients with past but not active EIMs at baseline; no change, worsened or improved for patients with active EIMs at baseline. Week 8 rates of remission (total Mayo score of ⩽2, with no individual subscore >1 and a rectal bleeding subscore of 0) and endoscopic improvement (Mayo endoscopic subscore of ⩽1; defined as ‘mucosal healing’ in the OCTAVE protocols), stratified by the presence of prior or active EIMs (as determined by the investigator) at baseline were evaluated. Remission, endoscopic improvement and steroid-free remission (remission, in addition to not requiring any treatment with corticosteroids for at least 4 weeks prior to the visit) were evaluated at week 52 in OCTAVE Sustain.
Statistical analysis
Analyses were performed in the full analysis set (FAS), defined as all randomly assigned patients, and were based on patients with non-missing data in OCTAVE Induction 1 and 2, and OCTAVE Sustain. For rates of remission, endoscopic improvement, and steroid-free remission, treatment differences (difference from placebo) and 95% confidence intervals (CIs) were calculated using normal approximation, and p values were based on chi-squared test by subgroups of EIM (yes/no) at baseline. For efficacy outcomes derived from the total Mayo score, non-responder imputation (NRI) was used for imputation of missing data. Patients were treated as non-responders after the time of discontinuation up to the visit they would have reached if they had stayed in the study.
Results
OCTAVE Induction 1 and 2
In total, 1139 patients were randomly assigned to receive treatment (tofacitinib 10 mg BID, n = 905; placebo, n = 234) in OCTAVE Induction 1 and 2. Four patients with missing data were excluded from the analysis of EIMs. Of the patients with non-missing data (N1 = 1135), 307 (27.0%) had any history of EIMs at baseline (Table 1). The most frequently reported active EIMs were peripheral arthritis (11.2%), sacroiliitis (1.0%), and oral ulcers/stomatitis (0.7%; Table 1). Four patients (0.4%) were classified as having an active thromboembolic disorder at induction baseline, as reported by the investigator (Table 1). All of these patients had a history of prior thromboembolic events that were being controlled by medication. One patient had a past medical history of deep vein thrombosis (DVT), and ongoing ‘venous insufficiency’ and was receiving tinzaparin as prophylaxis for DVT. One patient with a present medical history of venous thrombosis, hypertension, and left ventricular hypertrophy was receiving concomitant medication (amlodipine, atenolol, and irbesartan) prior to and during the study. One patient with a medical history of lower limb vein thrombosis was receiving enoxaparin sodium prior to and during the study for low leg phlebothrombosis. One patient with a present medical history of central venous thrombosis was receiving warfarin prior to and during the study for cerebral venous sinus thrombosis. A full description of thromboembolic events occurring during the tofacitinib UC clinical program have been reported previously.19
Table 1.
Summary of prior or active EIMs at baseline in OCTAVE Induction 1 and 2, and OCTAVE Sustain.
| EIM | OCTAVE Induction 1 and 2 (N1 = 1135) | OCTAVE Sustain (N1 = 591) | |
|---|---|---|---|
| History (prior/active) of EIM,a n (%) | 307 (27.0) | 53 (9.0) | |
| EIM by category, n (%) | Prior, but not currently active | Currently active | Currently active |
| Peripheral arthritis | 79 (7.0) | 127 (11.2) | 21 (3.6) |
| Oral ulcer/stomatitis | 71 (6.3) | 8 (0.7) | 2 (0.3) |
| Erythema nodosum | 37 (3.3) | 3 (0.3) | 1 (0.2) |
| Thromboembolic disorderb | 26 (2.3) | 4 (0.4)c | 2 (0.3) |
| Uveitis/iritis | 17 (1.5) | 3 (0.3) | 2 (0.3) |
| Sacroiliitis | 9 (0.8) | 11 (1.0) | 4 (0.7) |
| Pyoderma gangrenosum | 9 (0.8) | 2 (0.2) | 0 (0.0) |
| Myopathy | 6 (0.5) | 4 (0.4) | 0 (0.0) |
| Scleritis/episcleritis | 7 (0.6) | 1 (0.1) | 1 (0.2) |
| Ankylosing spondylitis | 2 (0.2) | 5 (0.4) | 0 (0.0) |
Data are based on the number of patients with non-missing data.
The number (%) of patients who reported any EIM (prior or active) in each study. Some patients reported more than one EIM.
As reported by the investigator. Past medical history of thromboembolic events collected.
As reported by the investigator.
DVT, deep vein thrombosis; EIM, extraintestinal manifestation; n, number of patients in each category; N1, number of patients with non-missing data.
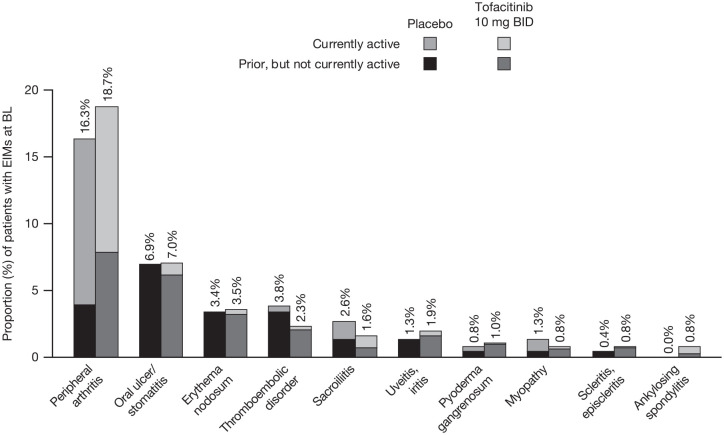
In OCTAVE Induction 1 and 2, similar proportions of patients had prior or active EIMs at baseline across treatment groups, the most common being peripheral arthritis (Figure 1). At baseline, 10.9% (98/902) of patients in the tofacitinib 10 mg BID group had currently active peripheral arthritis and 7.8% (70/902) of patients had prior, but not currently active, peripheral arthritis, compared with 12.4% (29/233) and 3.9% (9/233) of patients, respectively, in the placebo group. Two patients enrolled into OCTAVE Induction 1 and 2 had a medical history of primary sclerosing cholangitis.
Figure 1.
EIMs experienced by patients at baseline by treatment group in OCTAVE Induction 1 and 2.
Percentages represent the proportion of patients with prior or active EIMs at OCTAVE Induction baseline in placebo (N1 = 233) or tofacitinib 10 mg BID (N1 = 902) groups.
BID, twice daily; BL, baseline; EIM, extraintestinal manifestation; N1, number of patients with non-missing data.
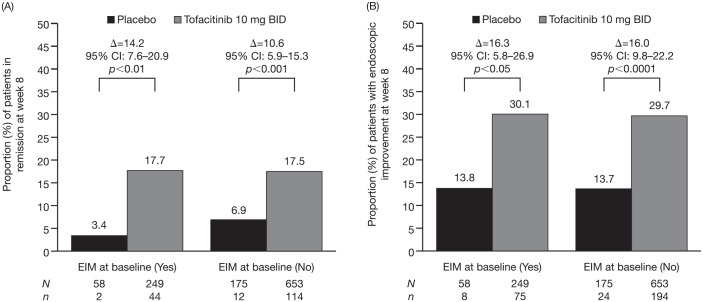
For remission at week 8 [Figure 2(A)], a statistically significant effect of treatment with tofacitinib 10 mg BID versus placebo was observed in patients with any history of EIMs at baseline [difference from placebo 14.2% (95% CI 7.6–20.9); p < 0.01] and without any history of EIMs at baseline [10.6% (95% CI 5.9–15.3); p < 0.001]. For endoscopic improvement at week 8 [Figure 2(B)], a statistically significant effect of treatment with tofacitinib 10 mg BID versus placebo was observed in patients with any history of EIMs at baseline [difference from placebo 16.3% (95% CI 5.8–26.9); p < 0.05] and without any history of EIMs at baseline [16.0% (95% CI 9.8–22.2); p < 0.0001]. The proportions of patients who achieved remission or endoscopic improvement were numerically similar within treatment groups, regardless of any history of EIMs at baseline.
Figure 2.
Proportion of patients in remission (A), and with endoscopic improvement (B) at week 8 in OCTAVE Induction 1 and 2, by EIM status at baseline (FAS, NRI, central read).
Δ, difference from placebo; BID, twice daily; CI, confidence interval; EIM, extraintestinal manifestation; FAS, full analysis set; n, number of patients with efficacy response; N, number of evaluable patients; NRI, non-responder imputation.
Change from baseline in EIMs in OCTAVE Induction 1 and 2
For all EIMs, other than peripheral arthritis and oral ulcers/stomatitis, the number of patients reporting an improvement, worsening, or new occurrence at week 8 of OCTAVE Induction 1 and 2 was less than five.
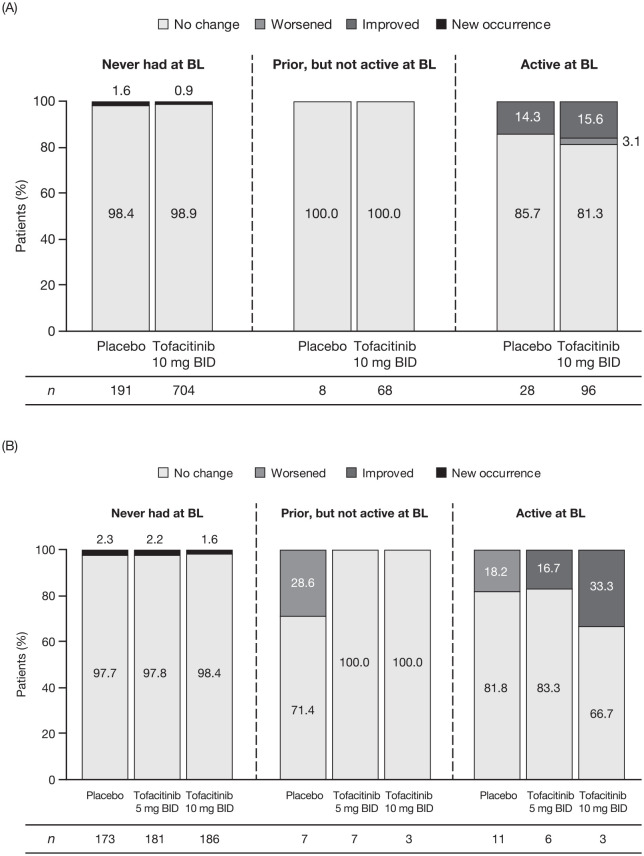
Similar proportions of patients with baseline active peripheral arthritis reported no change [tofacitinib 10 mg BID, 78/96 (81.3%); placebo, 24/28 (85.7%)] or an improvement [tofacitinib 10 mg BID, 15/96 (15.6%); placebo, 4/28 (14.3%)] from baseline at week 8. Three (3.1%) tofacitinib-treated patients experienced worsening of symptoms [Figure 3(A)]. These three patients with worsening of symptoms all received corticosteroids prior to enrollment into OCTAVE Induction 1 and 2 and continued to receive concomitant corticosteroids during the study. In patients who never had peripheral arthritis at baseline, there were nine new occurrences [tofacitinib 10 mg BID, 6/704 (0.9%); placebo, 3/191 (1.6%)] [Figure 3(A)].
Figure 3.
Peripheral arthritis: change from OCTAVE Induction baseline at week 8 or early termination (A) and change from OCTAVE Sustain baseline at week 52 or early termination (B), by EIM status.
BID, twice daily; BL, baseline; EIM, extraintestinal manifestation; n, number of patients in the specified category with non-missing values at week 8/early termination or week 52/early termination.
For oral ulcers/stomatitis, improvements were observed in three [tofacitinib 10 mg BID, 3/8 (37.5%)] tofacitinib-treated patients with active symptoms at baseline. Worsening symptoms were observed in two [2/16 (12.5%)] placebo-treated patients with a prior history (but no active symptoms at baseline) and there were six new occurrences in patients who had never had oral ulcers/stomatitis at baseline [tofacitinib 10 mg BID, 5/807 (0.6%); placebo, 1/211 (0.5%)]. See Supplemental Table 1 for a full list of new occurrences of EIMs.
OCTAVE Sustain
Overall, 592 patients with clinical response at week 8 of OCTAVE Induction 1 and 2 were randomly assigned to receive treatment in OCTAVE Sustain (tofacitinib 5 mg BID, n = 198; tofacitinib 10 mg BID, n = 196; placebo, n = 198). One patient (randomly assigned to receive tofacitinib 10 mg BID) with missing data was excluded from the analysis (N1 = 591). In total, 53 (9.0%) patients had any history of EIMs at baseline. The most frequently reported active EIMs at baseline of OCTAVE Sustain were peripheral arthritis (3.6%) and sacroiliitis (0.7%; Table 1). A greater proportion of patients had active peripheral arthritis at baseline of OCTAVE Induction 1 and 2 compared with baseline of OCTAVE Sustain.
In OCTAVE Sustain, the proportions of patients with active peripheral arthritis at baseline were higher in the placebo [12/198 (6.1%)] and tofacitinib 5 mg BID [6/198 (3.0%)] groups versus the tofacitinib 10 mg BID group [3/195 (1.5%)]. Similar proportions of patients in each treatment group were observed for other active EIMs at baseline (Table 2).
Table 2.
EIMs experienced by patients at baseline by treatment group following re-randomization into OCTAVE Sustain.
| EIM | Proportions of patients with active EIM at baseline of OCTAVE Sustain | ||
|---|---|---|---|
| EIM by category, n (%) | Placebo (N1 = 198) | Tofacitinib 5 mg BID (N1 = 198) | Tofacitinib 10 mg BID (N1 = 195) |
| Peripheral arthritis | 12 (6.1) | 6 (3.0) | 3 (1.5) |
| Oral ulcer/stomatitis | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Erythema nodosum | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Thromboembolic disordera | 1 (0.5) | 0 (0.0) | 1 (0.5) |
| Uveitis/iritis | 1 (0.5) | 0 (0.0) | 1 (0.5) |
| Sacroiliitis | 1 (0.5) | 3 (1.5) | 0 (0.0) |
| Scleritis/episcleritis | 0 (0.0) | 0 (0.0) | 1 (0.5) |
Percentages represent the proportion of patients with active EIMs at OCTAVE Sustain baseline in placebo (N1 = 198), tofacitinib 5 mg BID (N1 = 198), and tofacitinib 10 mg BID (N1 = 195) groups. No patients had active pyoderma gangrenosum, myopathy, or ankylosing spondylitis at OCTAVE Sustain baseline.
As reported by the investigator. Past medical history of thromboembolic events collected.
BID, twice daily; EIM, extraintestinal manifestation; n, number of patients in each category; N1, number of patients with non-missing data.
Two patients (0.3%) were classified as having an active thromboembolic disorder at maintenance baseline. This included one patient (with active thromboembolic disorder at induction baseline) with a present medical history of venous thrombosis, hypertension, and left ventricular hypertrophy who was receiving concomitant medication (amlodipine, atenolol, and irbesartan) prior to and during the study and one patient with a medical history of lower limb vein thrombosis who was receiving rivaroxaban prior to and during the study for lower leg phlebothrombosis.
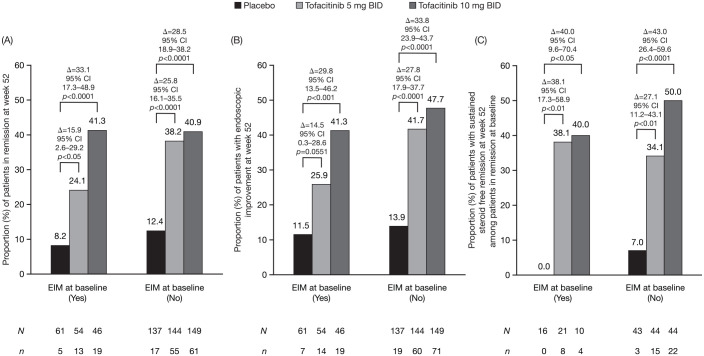
For remission and endoscopic improvement at week 52, a statistically significant effect of treatment with tofacitinib 10 mg BID versus placebo was observed for patients with and without any history of EIMs at baseline [Figure 4(A) and (B)]. Treatment effects of tofacitinib 5 mg BID versus placebo were statistically significant for remission in patients with and without any history of EIMs, and for endoscopic improvement in patients without any history of EIMs. Of patients in remission at baseline of OCTAVE Sustain, steroid-free sustained remission was achieved at week 52 in significantly more patients treated with tofacitinib 5 and 10 mg BID versus placebo, regardless of the history of active EIMs at baseline [Figure 4(C)].
Figure 4.
Proportion of patients in remission (A), with endoscopic improvement (B), and sustained steroid-free remission (C), at week 52 in OCTAVE Sustain, by EIM status at baseline (FAS, NRI, central read).
Δ, difference from placebo; BID, twice daily; CI, confidence interval; EIM, extraintestinal manifestation; FAS, full analysis set; N, number of evaluable patients; n, number of patients with efficacy response; NRI, non-responder imputation.
Change from baseline in EIMs in OCTAVE Sustain
In OCTAVE Sustain, most patients with active peripheral arthritis at baseline experienced no change at week 52 [tofacitinib 5 mg BID, 5/6 (83.3%); tofacitinib 10 mg BID, 2/3 (66.7%); placebo, 9/11 (81.8%)] [Figure 3(B)]. Two tofacitinib-treated patients [tofacitinib 5 mg BID, 1/6 (16.7%); tofacitinib 10 mg BID, 1/3 (33.3%)] experienced an improvement at week 52. Two [2/11 (18.2%)] placebo-treated patients reported a worsening of symptoms [Figure 3(B)]. In OCTAVE Sustain, for patients who had a prior history of peripheral arthritis but had no symptoms at baseline, worsening symptoms occurred in two [2/7 (28.6%)] placebo-treated patients and no tofacitinib-treated patients. Overall, 11 patients who had never had peripheral arthritis at baseline reported a new occurrence during OCTAVE Sustain [tofacitinib 5 mg BID, 4/181 (2.2%); tofacitinib 10 mg BID, 3/186 (1.6%); placebo, 4/173 (2.3%)]. For all other EIMs, the number of patients reporting an improvement, worsening, or new occurrence at week 52 of OCTAVE Sustain was ⩽3. See Supplemental Table 1 for further details of new occurrences of EIMs.
Discussion
EIMs in patients with UC are frequent and can be disabling, hence their management represents a challenge in clinical practice. In this post-hoc analysis, we investigated the efficacy of tofacitinib on remission and endoscopic improvement in patients with EIMs, and the impact of tofacitinib treatment on underlying EIMs in patients with moderately to severely active UC enrolled in the OCTAVE program. The EIMs were predefined and data were collected in a prospective fashion from all patients enrolled in the OCTAVE program. In OCTAVE Induction 1 and 2, and OCTAVE Sustain, 27.0% and 9.0% of patients had any history of EIMs at baseline, respectively. Consistent with previous reports, the most common EIM observed was peripheral arthritis.1,20 Among patients with UC, peripheral arthritis does not tend to cause bone erosion or deformity, unlike psoriatic arthritis and other inflammatory arthropathies.21
The presence of EIMs has previously been reported to increase the risk of poor clinical outcomes, such as lower remission rates.4 During induction and maintenance therapy, patients with and without any history of EIMs at baseline were shown to respond to tofacitinib treatment, with numerically similar proportions of patients receiving tofacitinib 10 mg BID achieving remission and endoscopic improvement. Furthermore, treatment effects of tofacitinib 10 mg BID (i.e. differences from placebo) were numerically similar for patients with and without any history of EIMs at baseline. Among patients receiving tofacitinib 5 mg BID with any history of EIMs at baseline in OCTAVE Sustain, a numerically lower proportion achieved remission and endoscopic improvement versus patients receiving tofacitinib 10 mg BID, and versus patients without any history of EIMs at baseline of OCTAVE Sustain. However, the proportion of patients in the tofacitinib 5 mg BID treatment group who achieved these efficacy endpoints was significantly higher than in the placebo group, regardless of any history of EIM at baseline of OCTAVE Sustain. While a greater proportion of patients had active peripheral arthritis at OCTAVE Induction baseline compared with OCTAVE Sustain baseline, this could be due to study design; whereby only patients with clinical response at week 8 of OCTAVE Induction 1 and 2 were enrolled into OCTAVE Sustain, potentially selecting out patients with more refractory disease and associated EIMs.
TNFis are thought to be effective for the treatment of EIMs, and a systematic review concluded that they are beneficial for the treatment of musculoskeletal, cutaneous, and ocular manifestations.11 Despite being beneficial in the treatment of EIMs, it is accepted that treatment with TNFis can induce paradoxical inflammation which is considered a drug-class effect of TNFis and is usually reversible on drug cessation.3 During OCTAVE Induction 1 and 2 and OCTAVE Sustain, few new occurrences of EIMs were reported. For patients who had never had peripheral arthritis at baseline, similar proportions of new occurrences were observed across treatment groups. Of note, corticosteroid tapering was mandatory during OCTAVE Sustain. Previous studies of EIMs in patients treated with vedolizumab reported an association between corticosteroid withdrawal and worsening of EIMs, with an increase in the incidence of arthritis/arthralgia in patients receiving corticosteroids at baseline in the weeks following corticosteroid decrease.22
The best way to reduce the burden of many EIMs is to treat the underlying bowel inflammation associated with UC; however, not all EIMs parallel intestinal disease activity.23 Here, our observations demonstrated that for patients with active peripheral arthritis at baseline, similar proportions of tofacitinib and placebo-treated patients reported an improvement or no change in symptoms by week 8 of OCTAVE Induction. In contrast, by week 52 of OCTAVE Sustain, improvements were only observed in tofacitinib-treated patients. These findings indicate that longer treatment periods may be required to observe more improvements in symptoms of peripheral arthritis with tofacitinib treatment versus placebo. The number of patients with active peripheral arthritis at baseline of OCTAVE Sustain was low; therefore, these results should be interpreted with caution.
The effectiveness of TNFis (infliximab, adalimumab, and certolizumab pegol) on EIM evolution was assessed in patients in the Swiss IBD Cohort Study.24 TNFi treatment was more frequent among patients with EIMs versus those without EIMs, with infliximab most commonly prescribed. Clinical response of underlying EIMs was observed in approximately 72% of patients, with the greatest response seen for psoriasis (100%), erythema nodosum (80%), aphthous stomatitis (78%), peripheral arthritis (73%), and uveitis (72%).24 The benefit of tofacitinib treatment for EIMs in UC has recently been demonstrated in a case report in which a patient with UC and inflammatory arthritis, who progressed on vedolizumab, was observed to achieve remission of inflammatory arthritis with extended release tofacitinib plus vedolizumab.25 In a separate case report, a patient with UC and spondyloarthropathy, who had experienced neurological complications following infliximab treatment, was treated with tofacitinib in combination with vedolizumab, and achieved clinical remission of both their UC and rheumatological symptoms.26
The post-hoc analysis of data from OCTAVE Induction 1 and 2 presented here is subject to some limitations. Patient numbers were low and did not permit a conclusion on the effect of tofacitinib on EIM symptoms. Due to protocol design, any history of overall EIMs includes patients with both prior and active EIMs. The collection of data via a pre-defined EIM list did not include a specific arthralgia category, distinct from the peripheral arthritis category. Arthralgia, joint pain in the absence of inflammation, is also common in patients with IBD and may be partly due to an initial adverse reaction associated with thiopurine therapy or the withdrawal of corticosteroids.21 There is currently no standardized approach for evaluating EIMs in UC clinical trials, making data interpretation and comparisons to different therapies difficult.27 Data on EIMs were collected at baseline of OCTAVE Induction 1 and 2 and updated at baseline of OCTAVE Sustain, but patients were not assessed by board certified experts in those particular conditions. Arthropathies were not adjudicated by a rheumatologist and therefore the type and cause could be challenging to diagnose accurately. Analysis of how EIM responses relate to intestinal outcomes would provide a worthwhile extension of the current research.
In conclusion, this analysis demonstrated that the incidence of EIMs at baseline in OCTAVE Induction 1 and 2 was consistent with previous reports in patients with UC. Patients with and without EIMs were shown to respond to tofacitinib treatment with generally similar treatment effects on UC endpoints. The most common active EIM was peripheral arthritis, for which many patients in OCTAVE Induction 1 and 2 and OCTAVE Sustain, reported improvement or no change from baseline with tofacitinib treatment. Further studies are warranted to elucidate more fully the effectiveness of tofacitinib, especially with extended use beyond induction, for carefully defined EIMs in patients with UC.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848211005708 for Extraintestinal manifestations at baseline, and the effect of tofacitinib, in patients with moderate to severe ulcerative colitis by David T. Rubin, Walter Reinisch, Thomas Greuter, Paulo G. Kotze, Marcia Pinheiro, Rajiv Mundayat, Eric Maller, Marc Fellmann, Nervin Lawendy, Irene Modesto, Stephan R. Vavricka and Gary R. Lichtenstein in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors would like to thank the patients, investigators, and study teams involved in the OCTAVE trials. The authors would like to thank Jerome Paulissen, an employee of Syneos Health, for providing statistical support. These studies were sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Helen Findlow, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461–464).
Footnotes
Authors’ note: Data in this manuscript were originally presented at the Annual Advances in Inflammatory Bowel Diseases (AIBD) congress, Orlando, FL, USA, 12–14 December 2019, and at the Semana Brasileira do Aparelho Digestivo (SBAD) congress, São Paulo, Brazil, 17–20 November 2018.
Conflict of interest statement: DTR has received consulting fees from AbbVie, AbGenomics, Allergan, Biomica, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Check-Cap Ltd., Dizal Pharma, Galen Pharma, Genentech, Gilead Sciences, GlaxoSmithKline, Ichnos Sciences, InDex Pharmaceuticals, Janssen, Lilly, Narrow River Management, Pfizer Inc., Prometheus Laboratories, Reistone Biopharma, Shire, Takeda, and Techlab Inc.; and grant support from Takeda. WR has received research support from Abbott, AbbVie, AESCA, Centocor, Dr. Falk Pharma, Immundiagnostik, and MSD; lecture fees from Abbott, AbbVie, AESCA, Aptalis, Celltrion, Centocor, Danone, Dr. Falk Pharma, Elan, Ferring Pharmaceuticals, Immundiagnostik, Mitsubishi Tanabe Pharma, MSD, Otsuka, PDL, Pharmacosmos, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; and consulting fees from Abbott, AbbVie, AESCA, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia Biologics, Bioclinica, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Cellerix, Celltrion, Centocor, ChemoCentryx, Covance, Danone, Dr. Falk Pharma, Elan, Ferring Pharmaceuticals, Galapagos, Genentech, Gilead Sciences, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson and Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Mitsubishi Tanabe Pharma, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pfizer Inc., Pharmacosmos, Procter & Gamble, Prometheus Laboratories, Robarts Clinical Trials, Schering-Plough, Second Genome, SetPoint Medical, Takeda, Therakos, TiGenix, UCB, Vifor, Zyngenia, and 4SC. TG has a consultancy contract with Sanofi-Aventis; received a travel grant from Falk Pharma GmbH and Vifor; and an unrestricted research grant from Novartis. PGK has received consulting and speaker fees from AbbVie, Janssen, Pfizer Inc., and Takeda; and speaker fees from UCB. MP, RM, MF, NL, and IM are employees and stockholders of Pfizer Inc. EM was an employee of Pfizer Inc. at the time this research was conducted. SRV has received consulting fees and unrestricted research grants from Abbott, Ferring Pharmaceuticals, MSD, Pfizer Inc., Takeda, Tillotts, UCB, Vifor, and Falk Pharma GmbH. GRL has received research support and/or funding from Celgene, Janssen Orthobiotech, Pfizer Inc, Takeda, and UCB; consulting fees from AbbVie, American Regent, Cellceutix, Celgene, Eli Lilly, Endo Pharmaceuticals, Ferring Pharmaceuticals, Gilead Sciences, Janssen Orthobiotech, Merck, Morphic Therapeutics, Pfizer Inc., Prometheus Laboratories, Romark, Salix/Valeant, Shire, Takeda, and UCB; honoraria from the American College of Gastroenterology, American Regent, Gastroenterology and Hepatology, Merck, Romark, Springer Science and Business Media, and Up-To-Date; and royalties from Professional Communications Inc. and SLACK Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The clinical trials described in this article were sponsored by Pfizer Inc. Funding for medical writing support was provided by Pfizer Inc.
Data sharing statement: Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
ORCID iD: Paulo G. Kotze  https://orcid.org/0000-0002-9632-6691
https://orcid.org/0000-0002-9632-6691
Supplemental material: Supplemental material for this article is available online.
Contributor Information
David T. Rubin, University of Chicago Medicine, Inflammatory Bowel Disease Center, 5841 S. Maryland Avenue, MC4076, Chicago, IL 60637, USA.
Walter Reinisch, Medical University of Vienna, Vienna, Austria.
Thomas Greuter, Department of Gastroenterology and Hepatology, University Hospital Zürich, Zürich, Switzerland.
Paulo G. Kotze, IBD Outpatient Clinics, Colorectal Surgery Unit, Catholic University of Paraná (PUCPR), Curitiba, Brazil
Marcia Pinheiro, Pfizer Inc., São Paulo, Brazil.
Rajiv Mundayat, Pfizer Inc., New York, NY, USA.
Eric Maller, Pfizer Inc., Collegeville, PA, USA.
Marc Fellmann, Pfizer Switzerland AG, Zürich, Switzerland.
Nervin Lawendy, Pfizer Inc., Collegeville, PA, USA.
Irene Modesto, Pfizer Inc., New York, NY, USA.
Stephan R. Vavricka, Department of Gastroenterology and Hepatology, University Hospital Zürich, Zürich, Switzerland; Zentrum für Gastroenterologie und Hepatologie AG, Zürich, Switzerland.
Gary R. Lichtenstein, Division of Gastroenterology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
References
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017; 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Juillerat P, Manz M, Sauter B, et al. Therapies in inflammatory bowel disease patients with extraintestinal manifestations. Digestion. Epub ahead of print 17 February 2020. DOI: 10.1159/000502816 [DOI] [PubMed] [Google Scholar]
- 3. Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016; 10: 239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu W, Ou W, Guo Y, et al. Clinical outcomes and risk factors of secondary extraintestinal manifestation in ulcerative colitis: results of a multicenter and long-term follow-up retrospective study. PeerJ 2019; 7: e7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vavricka SR, Schoepfer A, Scharl M, et al. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2015; 21: 1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long MD. Overview of extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY) 2019; 15: 161–163. [PMC free article] [PubMed] [Google Scholar]
- 7. Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol 2006; 12: 4819–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol 2011; 106: 110–119. [DOI] [PubMed] [Google Scholar]
- 9. Hiller A, Biedermann L, Fournier N, et al. The appearance of joint manifestations in the Swiss inflammatory bowel disease cohort. PLoS One 2019; 14: e0211554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chams S, Badran R, Sayegh SE, et al. Inflammatory bowel disease: looking beyond the tract. Int J Immunopathol Pharmacol 2019; 33: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol 2017; 15: 25–36.e27. [DOI] [PubMed] [Google Scholar]
- 12. Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016; 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Food and Drug Administration. XELJANZ® (tofacitinib): highlights of prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=959 (2020, accessed 26 October 2020).
- 14. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 15. Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011; 186: 4234–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bao L, Zhang H, Chan LS. The involvement of the JAK–STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT 2013; 2: e24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banerjee S, Biehl A, Gadina M, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 2017; 77: 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vavricka SR, Galván JA, Dawson H, et al. Expression patterns of TNFα, MAdCAM1, and STAT3 in intestinal and skin manifestations of inflammatory bowel disease. J Crohns Colitis 2018; 12: 347–354. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Panés J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019; 50: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vavricka SR, Rogler G, Gantenbein C, et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss inflammatory bowel disease cohort. Inflamm Bowel Dis 2015; 21: 1794–1800. [DOI] [PubMed] [Google Scholar]
- 21. Annese V. A review of extraintestinal manifestations and complications of inflammatory bowel disease. Saudi J Med Med Sci 2019; 7: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Feagan BG, Sandborn WJ, Colombel J-F, et al. Incidence of arthritis/arthralgia in inflammatory bowel disease with long-term vedolizumab treatment: post hoc analyses of the GEMINI trials. J Crohns Colitis 2019; 13: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veloso FT. Extraintestinal manifestations of inflammatory bowel disease: do they influence treatment and outcome? World J Gastroenterol 2011; 17: 2702–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD Cohort Study. Inflamm Bowel Dis 2017; 23: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 25. Wang W, Cleveland NK, Ollech J, et al. Use of tofacitinib for the treatment of arthritis associated with ulcerative colitis. ACG Case Rep J 2019; 6: e00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Le Berre C, Loeuille D, Peyrin-Biroulet L. Combination therapy with vedolizumab and tofacitinib in a patient with ulcerative colitis and spondyloarthropathy. Clin Gastroenterol Hepatol 2019; 17: 794–796. [DOI] [PubMed] [Google Scholar]
- 27. Guillo L, D’Amico F, Serrero M, et al. Assessment of extraintestinal manifestations in inflammatory bowel diseases: a systematic review and a proposed guide for clinical trials. Unit Eur Gastroenterol J. Epub ahead of print 10 August 2020. DOI: 10.1177/2050640620950093 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848211005708 for Extraintestinal manifestations at baseline, and the effect of tofacitinib, in patients with moderate to severe ulcerative colitis by David T. Rubin, Walter Reinisch, Thomas Greuter, Paulo G. Kotze, Marcia Pinheiro, Rajiv Mundayat, Eric Maller, Marc Fellmann, Nervin Lawendy, Irene Modesto, Stephan R. Vavricka and Gary R. Lichtenstein in Therapeutic Advances in Gastroenterology