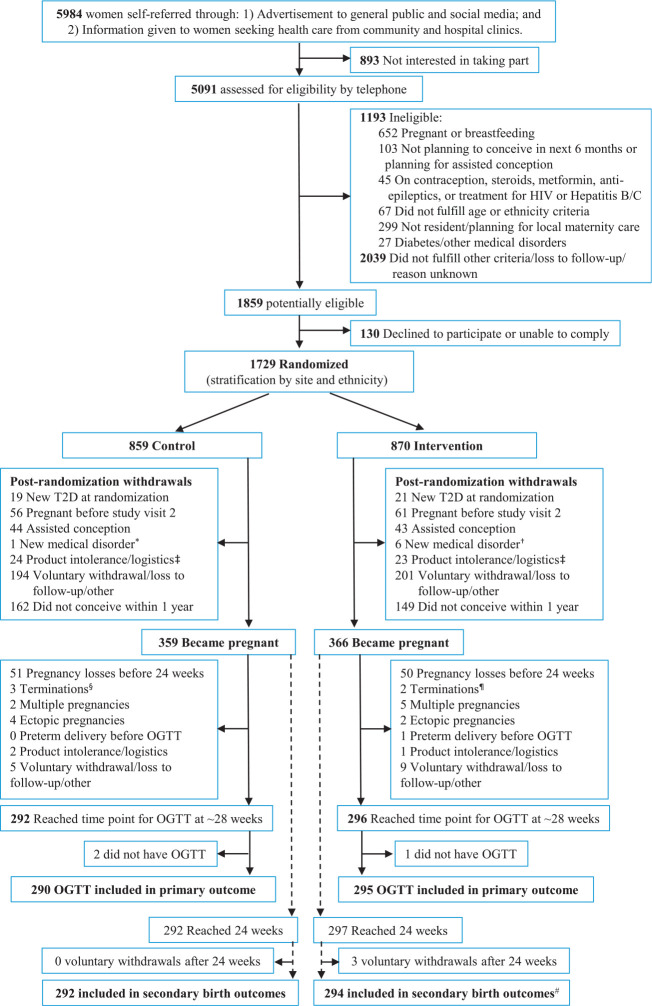
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram outlining participant flow. *Premature ovarian failure. †New-onset Graves’ disease, hemoglobinopathy with iron overload, prolactinoma, endometrial polyp, endometrial atypia, breast cancer. ‡Withdrew because product may contain animal remnants, no storage space in refrigerator, participant suspicion of product-related symptoms. §Includes two cases of trisomy 21, Klinefelter syndrome. ¶Includes hypoplastic left heart syndrome, unknown reason in private clinic. #Includes one stillbirth and one neonatal death. T2D, type 2 diabetes.