Fig. 4.
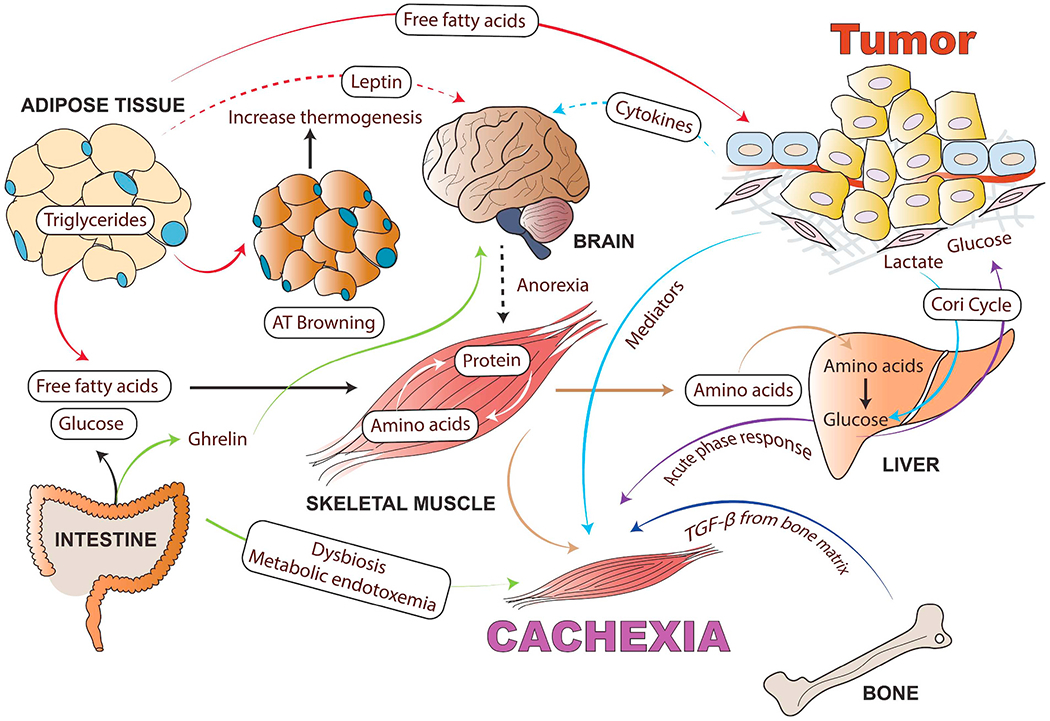
Cancer cachexia as a multi-organ metabolic syndrome: Although muscle and adipose tissue wasting are the main characteristics of cancer cachexia, tumor and host-derived factors and systemic inflammation also influence many other organs such as brain, liver, heart, bone, pancreas, cardiac muscle, and gut, which are involved in the cachectic process. Tumor-derived cytokines cause systemic inflammation in the hypothalamus and stimulate neuropeptides involved in the regulation of food intake. Anorexia exacerbates body wasting in cancer cachexia. In addition to tumor-derived mediators, adipokines, muscle-derived cytokines (myokines) and brain-derived anorexia factors also influence the wasting of skeletal muscle and AT as well as increase inflammation in the tumor microenvironment. This inter-tissue communication directly or indirectly influences tissue metabolism and the severity of the cachexia syndrome. Inflammatory cytokines can increase lipolysis in white adipose tissue (WAT), which releases free fatty acids that further fuel the tumor and facilitate muscle wasting. Cachexia can cause adipose tissue browning (WAT switches to brown adipose tissue) and increased thermogenesis. In turn, the myokines activate pathogenic mechanisms in the skeletal muscle and adipose tissue. Muscle wasting releases free amino acids resulting from active protein degradation during cancer cachexia, which drives the acute phase response in the liver. Cancer-associated gut microbiota dysfunction alters mitochondrial energy metabolism in skeletal muscle, contributing to the negative energy balance in cachectic cancer patients. In the case of cancer-mediated bone metastasis, increased activity of the osteoclasts results in the activation of TGF-β from the bone matrix, which can facilitate muscle wasting and reduce muscle strength. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
