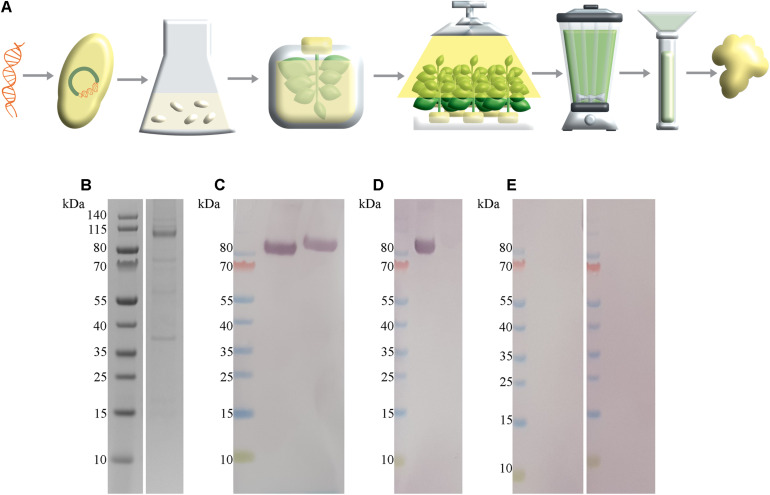
FIGURE 2.
SARS-CoV-2 S1-His antigen production workflow with corresponding size and functionality analysis. (A) Schematic overview about plant-based transient recombinant antigen production. SARS-CoV-2 S1 protein encoding DNA was transformed into Agrobacterium tumefaciens. Then, N. benthamiana plants were vacuum infiltrated with A. tumefaciens. After cultivation of transiently infiltrated plants for 5 days, leaves were harvested, followed by protein extracted via juicing and purification of the target protein using immobilized metal affinity chromatography (IMAC) purification strategy. (B) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of IMAC-purified SARS-CoV-2 S1-His antigen recombinant produced in N. benthamiana with expected size of 80 kDa, under reducing conditions. In total, approximately 328 ng SARS-CoV-2 S1 protein was loaded. (C) Western blot analysis of SARS-CoV-2 S1-His protein, probed with anti-His antibody under non-reducing conditions (left) and reducing conditions (right). (D) Western blot analysis of 2 μg SARS-CoV-2 S1-His protein probed with patient serum previously tested positive with Liaison SARS-CoV-2 S1/S2 IgG assay and (E) each probed with a patient sera tested negative for the occurrence of SARS-CoV-2-specific antibodies.