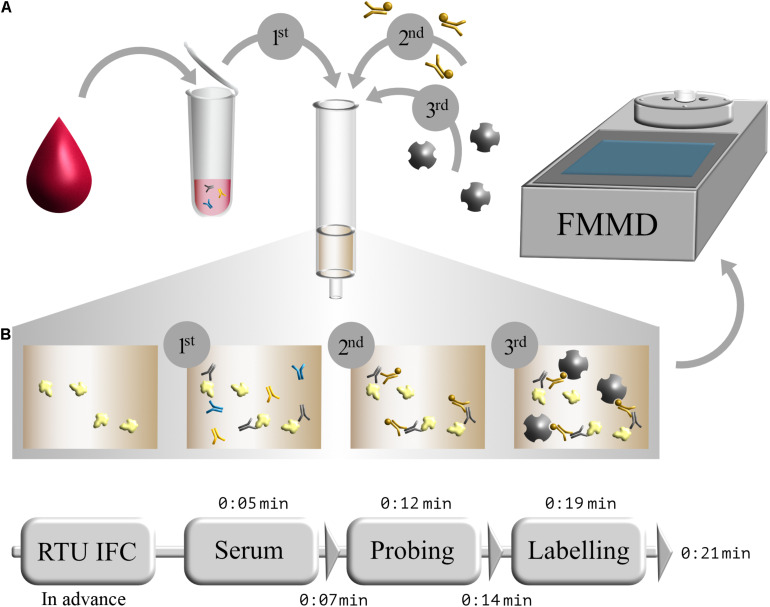
FIGURE 3.
Schematic workflow of serological magnetic immunodetection for detection of SARS-CoV-2-specific antibodies in human serum. (A) Initial application of human blood or serum onto SARS-CoV-2 S1-antigen precoated and blocked ready-to-use immunofiltration column (IFC; 1); sample is 40-fold prediluted. Following retention of antigen-specific antibodies within the matrix, IFC is washed and loaded with biotinylated human IgG-specific secondary antibody (2). Subsequently, IFC is washed again, and streptavidin-functionalized magnetic particles were applied (3), resulting in a magnetic labeling of bound antibody complex. Finally, IFC is washed again, and enriched magnetic particles were sensed by means of frequency magnetic mixing detection (FMMD) technology. (B) Chronological overview of magnetic immunodetection assay duration, starting with ready-to-use immunofiltration columns (RTU-IFCs), which can be coated and blocked in advance. For analysis of SARS-CoV-2-specific antibody occurrence in human serum, prediluted serum is applied and incubated for 5 min (Serum), followed by an subsequent washing step of 2 min (depicted as triangle). Afterward, biotinylated human IgG-specific secondary antibody is applied (Probing) and incubated for additional 5 min prior another 2 min washing step. Finally, magnetic beads are applied (Labeling), incubated for 5 min and washed for 2 min, resulting in an assay duration of in total 21 min. FMMD readout takes only about 10 s.