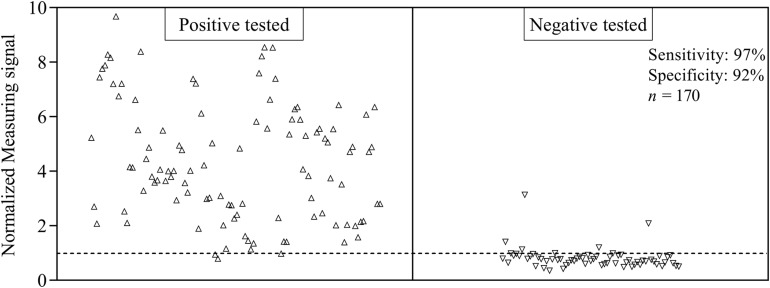
FIGURE 4.
Sensitivity and specificity of magnetic immunodetection-based SARS-CoV-2-specific antibody testing. A total of 170 patient sera were previously tested with certified DiaSorin Liaison SARS-CoV-2 S1/S2 IgG assay in clinical environment for the occurrence of SARS-CoV-2-specific antibodies. Afterward, sera were retested using magnetic immunodetection with SARS-CoV-2 S1-His protein coated and blocked ready-to-use immunofiltration columns (RTU-IFCs). After application of sera, followed by subsequent addition of biotinylated human IgG-specific secondary antibody and streptavidin-functionalized magnetic particles, IFCs were measured using a handheld frequency mixing magnetic detection (FMMD) readout device. Each data point represents a patient serum (n = 1). Dashed line indicates the threshold defining, whether sera were positive or negative tested using magnetic immunodetection.