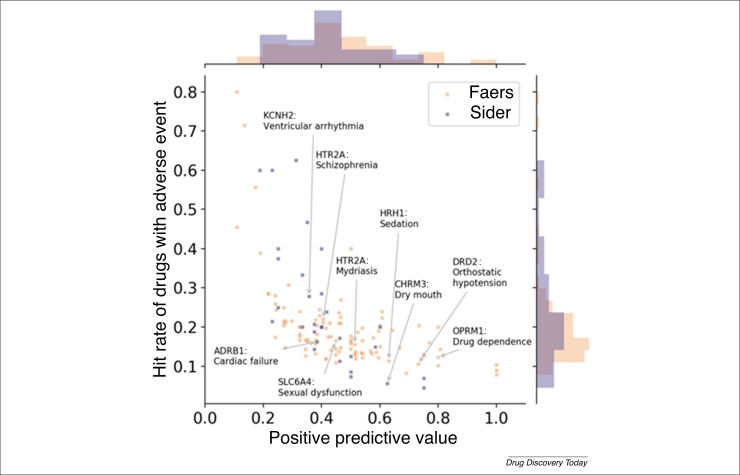
Figure 2.
The positive predictive values (PPV) of target–adverse event associations against the hit rate or recall (i.e., the fraction of drugs associated with the adverse event also being active at an individual protein target). Activity calls were made based on the ratio of the in vitro bioactivity and the unbound plasma concentration. Target–adverse event pairs with a high PPV tend to have a low hit rate, meaning only a small share of all drugs associated with the adverse event would be picked up by the bioactivity at the target. Alternatively, a high hit rate is associated with a low PPV, indicating a high false positive rate for that target–adverse event combination. Thus, overall, there exists no clear 1:1 relationship between on-target activity and observed adverse events after compound administration. Abbreviations: ADRA1B, α1b adrenergic receptor; ACE, angiotensin-converting enzyme; CHRM1/2/3, muscarinic acetylcholine receptor M1/2/3; PTGS1, cyclooxygenase-1; DRD2, dopamine D2 receptor; FAERS, US Food and Drug Administration Adverse Event Reporting System; HTR2A, serotonin 2a (5-HT2a) receptor; HTR2C, serotonin 2c (5-HT2c) receptor; KCNH2, hERG; SIDER, SIDe Effect Resource.