Investigations into γ- vs. δ-selectivity.
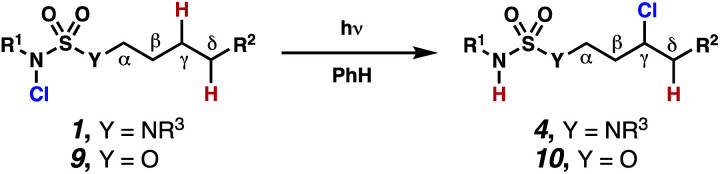
| |||||
|---|---|---|---|---|---|
| Entrya | Product | γ : δb | ΔΔG‡c (kcal mol−1) | Yieldd | |
| 1 |
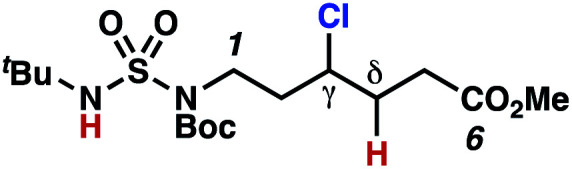
|
4k | —e | ≤−1.77f | 83 |
| 2 |
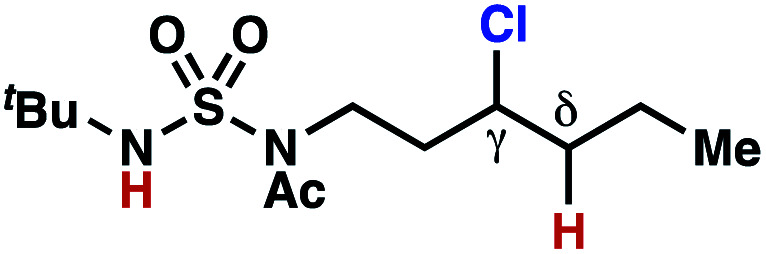
|
4l | >20 : 1 | ≤−1.77f | 87 |
| 3 |
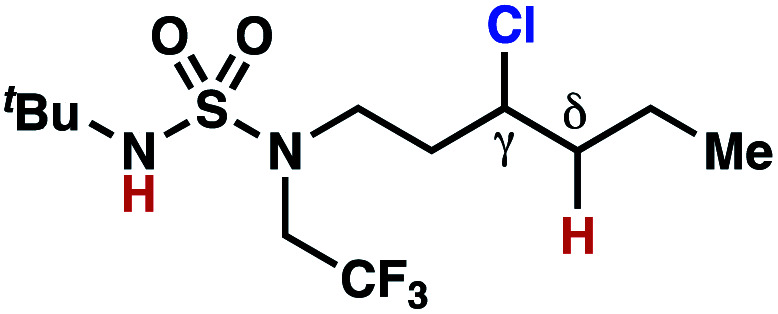
|
4m | —e | ≤−1.77f | 97 |
| 4 |
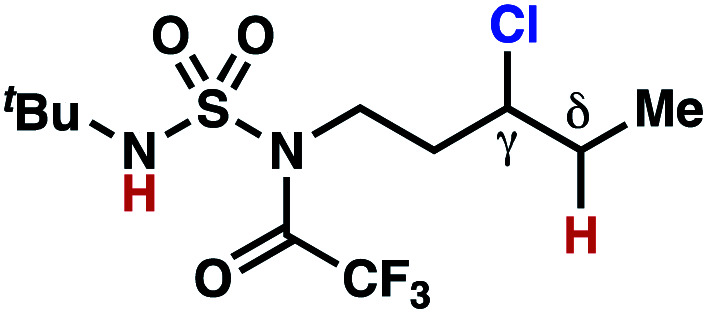
|
4n | —e | ≤−1.77f | 63 |
| 5 |
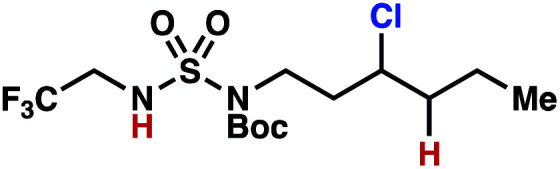
|
4o | 11 : 1 | −1.39 | 79 |
| 6 |
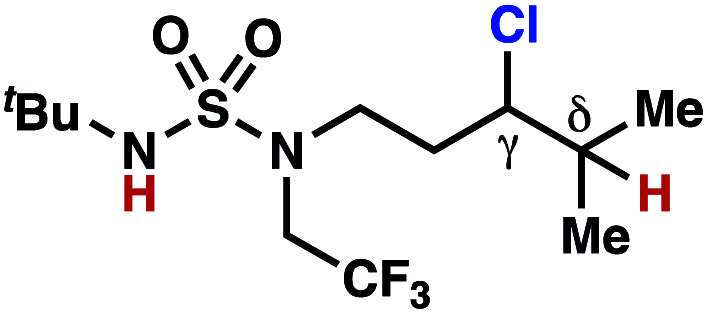
|
4p | 13 : 1 | −1.52 | 70 |
| 7 |
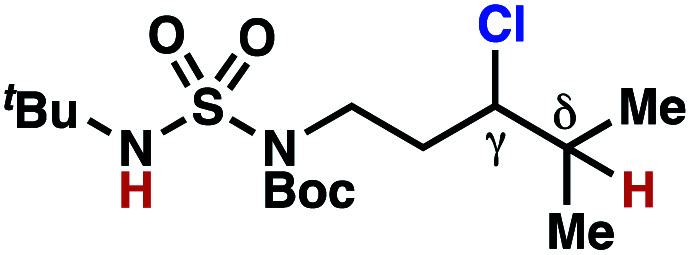
|
4q | 2 : 1 | −0.41 | 98g |
| 8 |
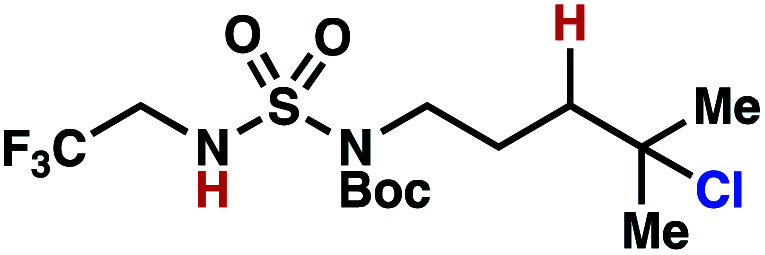
|
7r | 1 : 2 | +0.38 | 84g |
| 9h |
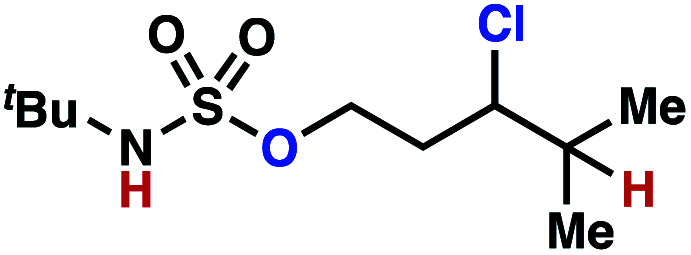
|
10a | 3 : 1 | −0.69 | 57g |
| 10h |
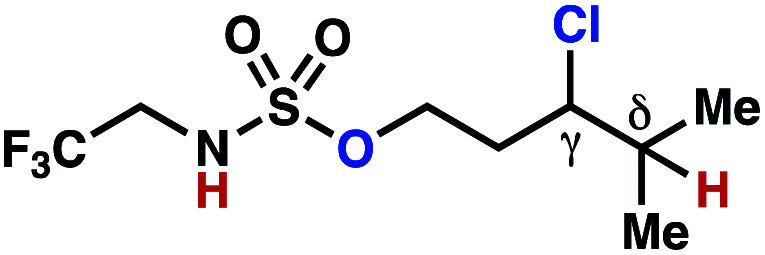
|
10b | 4 : 1 | −0.82 | 67g |
Conditions A.
Determined by 1H or 19F NMR of crude mixture.
Calculated from experimental product ratios.
Isolated yield of depicted product.
δ-Chlorinated-isomer not detected.
Calculated assuming ≥20 : 1 ratio of 4 : 7.
Isolated as a mixture of γ- and δ-chlorinated isomers.
Conditions: 1.0 equiv. N-chlorosulfamate 9, PhH (0.07 M), 2 blue Kessil lamps.11
