Comparison of calculated to experimental ΔΔG‡ values.
| Entry | Parent compound | Experimental ΔΔG‡a (kcal mol−1) | Calculated ΔΔG‡b (kcal mol−1) |
|---|---|---|---|
| 1 |
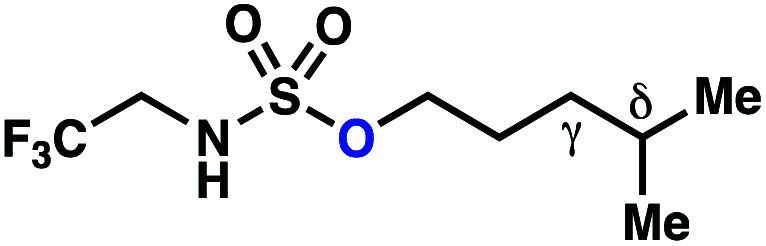
|
−0.82 | −3.33 |
| 2 |
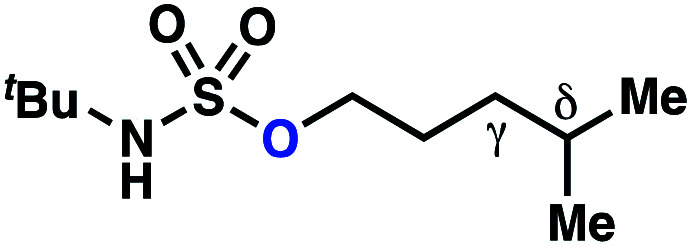
|
−0.69 | −1.09 |
| 3 |
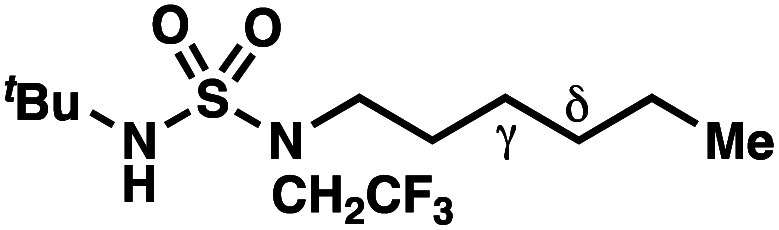
|
≤−1.77 | −3.74 |
| 4 |
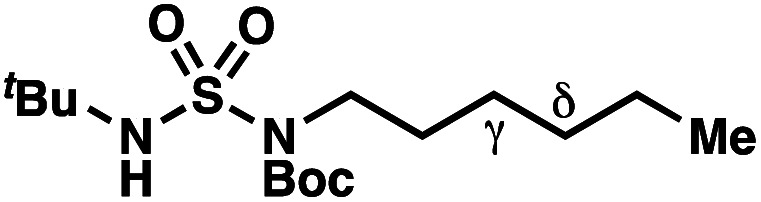
|
−1.56 | −2.98 |
| 5 |
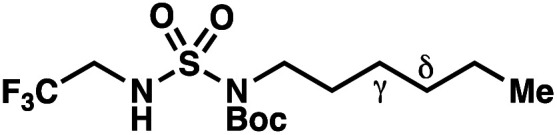
|
−1.39 | −2.06 |
| 6 |
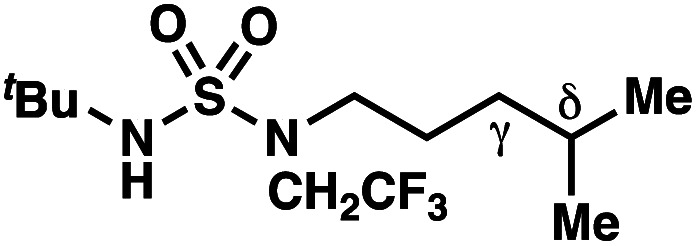
|
−1.52 | +0.35 |
| 7 |
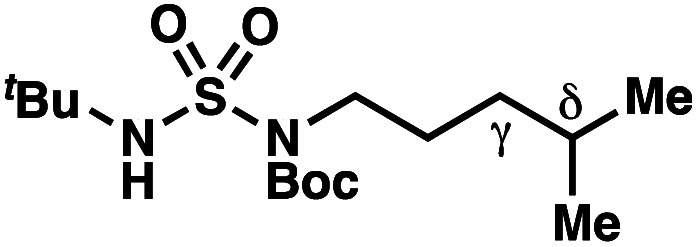
|
−0.41 | +2.24 |
| 8 |
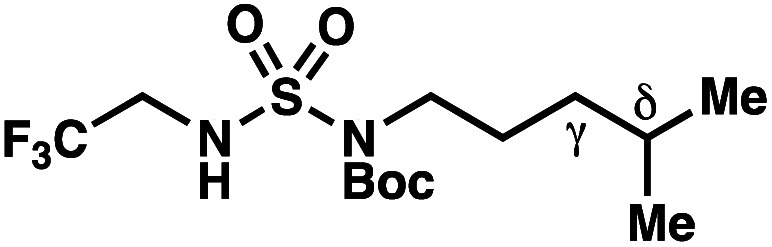
|
+0.38 | +1.04 |
ΔΔG‡ = −RT ln(γ-chlorinated product/δ-chlorinated product) ad determined by 1H or 19F NMR of crude reaction mixture.
ΔΔG‡ = (ΔG(1,6-HAT TS) − ΔG(1,7-HAT TS)) as determined from the calculated Gibbs free energies using uB3LYP/6-31+G(d,p).
