Supplemental Digital Content is available in the text.
Keywords: critical care, hyperoxia, injuries, intensive care units, oxygenation, trauma
Abstract
OBJECTIVES:
Hyperoxia is common among critically ill patients and may increase morbidity and mortality. However, limited evidence exists for critically injured patients. The objective of this study was to determine the association between hyperoxia and in-hospital mortality in adult trauma patients requiring ICU admission.
DESIGN, SETTING, AND PARTICIPANTS:
This multicenter, retrospective cohort study was conducted at two level I trauma centers and one level II trauma center in CO between October 2015 and June 2018. All adult trauma patients requiring ICU admission within 24 hours of emergency department arrival were eligible. The primary exposure was oxygenation during the first 7 days of hospitalization.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Primary outcome was in-hospital mortality. Secondary outcomes were hospital-free days and ventilator-free days. We included 3,464 critically injured patients with a mean age of 52.6 years. Sixty-five percent were male, and 66% had blunt trauma mechanism of injury. The primary outcome of in-hospital mortality occurred in 264 patients (7.6%). Of 226,057 patient-hours, 46% were spent in hyperoxia (oxygen saturation > 96%) and 52% in normoxia (oxygen saturation 90–96%). During periods of hyperoxia, the adjusted risk for mortality was higher with greater oxygen administration. At oxygen saturation of 100%, the adjusted risk scores for mortality (95% CI) at Fio2 of 100%, 80%, 60%, and 50% were 6.4 (3.5–11.8), 5.4 (3.4–8.6), 2.7 (1.7–4.1), and 1.5 (1.1–2.2), respectively. At oxygen saturation of 98%, the adjusted risk scores for mortality (95% CI) at Fio2 of 100%, 80%, 60%, and 50% were 7.7 (4.3–13.5), 6.3 (4.1–9.7), 3.2 (2.2–4.8), and 1.9 (1.4–2.7), respectively.
CONCLUSIONS:
During hyperoxia, higher oxygen administration was independently associated with a greater risk of mortality among critically injured patients. Level of evidence: Cohort study, level III.
Oxygen is frequently administered to critically injured patients to prevent hypoxia and enhance oxygen delivery (1, 2). This life-saving maneuver has undisputed importance to treat and prevent morbidity associated with hypoxia. However, excessive oxygen supplementation is routine and often results in hyperoxia (oxygen saturation [Spo2] > 96% or Pao2 > 150 mm Hg) (3–6).
Emerging evidence indicates that even modest hyperoxia be harmful. Supraphysiologic levels of oxygen in critically ill patients have repeatedly been associated with an increase in both morbidity and mortality (5–8). However, most studies focus exclusively on medical patients, as reflected by prior systematic reviews (2, 9–14). Limited evidence exists specifically regarding hyperoxia in trauma patients. Based on our review of the literature via a systematic Medical Subject Headings search, prior studies are limited to mostly traumatic brain injury (TBI) patients and do not account for cumulative oxygen exposure (15).
Beyond patient morbidity, the avoidance of hyperoxia may also improve resource utilization in both civilian and military contexts. Oxygen is a limited resource that is challenging to obtain in austere settings, including combat settings. It requires substantial resources, space, weight, and logistics to procure. Better understanding of the extent to which hyperoxia is not beneficial or even harmful could decrease this logistic burden in forward military operations and other austere settings.
In this study, we evaluated the association between hyperoxia and supplemental oxygen delivery with in-hospital mortality in critically injured patients. We hypothesized that increased time in hyperoxia would be associated with increased mortality. Clarifying this association has the potential to reduce morbidity, reduce mortality, and improve resource utilization in an underinvestigated field of trauma research.
MATERIALS AND METHODS
Patient records were obtained from regional trauma registries and electronic health records (EHRs) at two level I trauma centers (University of Colorado Hospital, Aurora, CO, and Memorial Hospital Center, Colorado Springs, CO) and one level II trauma center (Medical Center of the Rockies, Loveland, CO). All are University of Colorado Health hospitals, share a common reporting structure to the state of Colorado trauma registry and use the same EHR (Epic). Critically injured patients were defined as: 1) having an International Classification of Diseases, 10th Edition (ICD-10) code for injury/trauma, 2) meeting criteria for entry into the Colorado state trauma registry, and 3) requiring admission to the ICU. Admitting service was generally trauma and acute care surgery. We collected data from October 1, 2015, to June 30, 2018. This multicenter, retrospective cohort study was approved by the Colorado Multiple Institutional Review Board (number 17-1359) as a minimal risk study with waiver of informed consent.
Individuals without an injury code from the trauma registry definitions or who were coded as a readmission were excluded. Individuals who died in the hospital during the study period but who were missing a time of death were assigned a time of death of noon. Patients with suspected or confirmed carbon monoxide positioning—a condition which dictates intentional hyperoxia—were excluded based on ICD-10 coding. Longitudinal measurements of Fio2 (continuous), Spo2 (continuous), and Pao2 (Pao2) (intermittent) were obtained from entry to the emergency department through the first 7 ICU days. Baseline characteristics and illness severity were determined using time of intubation and extubation (if applicable), Acute Physiology and Chronic Health Evaluation (APACHE) II score, mechanism of injury (MOI), patient comorbidities, ventilator-free days (VFDs), and hospital-free days (HFDs). Intubation and extubation times were used to determine when a patient required a ventilator. When two intubation times occurred together without an interim extubation, the patient was assumed to be on a ventilator from the earlier time point. When two extubation times occurred together without an interim intubation, the patient was assumed to be on ventilator until the later time point. Manual chart review on a selected number of patients was performed to confirm this approach. There were no instances of more than two intubation/extubation times occurring together. Patients for whom their first procedure was an extubation were assumed to be ventilated from arrival and patients whose last procedure was an intubation were assumed to be ventilated until departure.
Spo2 measurements were paired to the nearest prior Fio2 measurement. Spo2 measurements were assumed to remain unchanged until the next Spo2 measurement was recorded. Measurements outside of the range 0–100% were discarded. Supplemental oxygen measurements recorded in liters per minute (L/min) were converted to Fio2 using the formula x × 3.5 + 21 where “x” is the flow rate in L/min (16–19). Fio2 measurements outside of the range 21–100% were discarded. Fio2 measurements from the ventilator setting flow sheets were assumed to be remain constant until the patient was extubated or a new setting was recorded. Supplemental oxygen was assumed to remain constant until the next record (either Fio2, supplemental oxygen, or room air) or for 12 hours. No value of Fio2 was assumed for the first 12 hours of a patient’s visit in the absence of a recorded value. After 12 hours with no record, the patient was assumed to be on room air, regardless of mechanical ventilation status. All vital signs were obtained via electronic capture from the EHR. Safe upper limits in critically ill patients without trauma are 96% Spo2 and 150 mm Hg Pao2 (4–6). Based on a recent Delphi Consensus investigating oxygen targets for trauma patients, hypoxia was defined as Spo2 less than 88%, mild hypoxia as Spo2 88–89%, normoxia as Spo2 90–96%, and hyperoxia as Spo2 greater than 96% (20).
We calculated APACHE scores from the patient’s worst value in the first 24 hours (21). APACHE physiologic variables with no record were assigned a value of zero. For the oxygenation component, if Pao2 was completely missing for an individual, the worst serum bicarbonate (Hco3) was used. If any Fio2 value greater than 50% was recorded in the first 24 hours, the worst alveolar–arterial oxygen gradient was used. Otherwise, the worst Pao2 was used.
We classified comorbidities using ICD-10 codes according to classifications provided by the Agency for Healthcare Research and Quality (22). Four cardiopulmonary comorbidities were flagged: congestive heart failure, valvular disease, pulmonary circulation disorders, and peripheral vascular disorders. The sum of a patient’s other noncardiopulmonary comorbidities was also used as a covariate in the analysis. All included comorbidities are listed in Supplemental Table 1 (http://links.lww.com/CCX/A606).
We categorized MOI using ICD-10 codes according to classifications provided by the Centers for Disease Control and Prevention (23). If more than one category was present in the ICD-10 code, preference was given in the following order: penetrating, motor vehicle, fall, or other. MOI was then dichotomized to blunt (fall, motor vehicle) and nonblunt (penetrating and other) for modeling purposes.
We calculated VFDs for the first 28 days of a patient’s hospital stay. Following published literature (24–26), days were counted toward invasive mechanical ventilation if any of the following conditions were met: 1) the patient was mechanically ventilated within the first 28 days of ICU admission, 2) the patient was reintubated within 48 hours of attempted extubation, 3) the patient had a tracheostomy and required noninvasive ventilation, or 4) the patient had a tracheostomy and was unable to tolerate a lack of invasive/noninvasive ventilation for greater than 48 hours. If the patient was hospitalized for less than 28 days, all days after they were discharged were considered ventilator free. Patients who were never on a ventilator were assigned a value of 28 (24, 27). We counted days where a patient was only on a ventilator in the operating room as ventilator free. We defined HFDs as 90 days minus the length of stay in the hospital in days.
We analyzed the primary outcome, in-hospital mortality, using a Cox proportional hazard regression with time-varying covariates. Spo2 and Fio2 were evaluated at all time points in each patient record. We assessed linear versus nonlinear relationships and found nonlinear to be the most adequate fit (Supplemental Fig. 1, http://links.lww.com/CCX/A607). Quadratic terms were used for both Spo2 and Fio2, along with their interaction, to accommodate for a nonlinear relationship. Estimation of all Cox models was performed with the “survival” package v.2.44.1.1 in R v3.5.2 (Vienna, Austria). Marginal risk scores were calculated using the ggeffects package v.0.9.0 in R v3.5.2. Risk scores are calculated as the ratio of the exponentiated linear predictor with specified values of the oxygenation variables and reference values for all other covariates to the exponentiated linear predictor for a hypothetical patient with mean values for all predictors included in the model.
We also conducted a secondary analysis of VFDs and HFDs. The primary exposure variables were percentage of time in hyperoxia (Spo2 > 96%) and percentage of time on room air (Fio2 = 21%). To account for violations of linear regression assumptions (zero inflation, right censoring) as well as the possibility of death, we used multinomial regression models to estimate the association between outcomes and exposure variables. Subjects who died were assigned to a different category than those with zero VFDs and HFDs. Further categories were created based on the distribution of the data and the available sample size, balancing the intention of avoiding excessive sparse categories with interpretability considerations. The sample size for 22 through 28 VFDs was large enough to allow inference at the day level. We grouped remaining VFDs into three weekly categories: days 1–7, days 8–14, and days 15–21. We grouped HFDs days 1–55, days 56–62, days 63–69, days 70–76, days 77–83, and days 84–90. We adjusted all models for age, race/ethnicity, sex, smoking status, APACHE II score, presence of cardiopulmonary comorbidities, number of other comorbidities, and MOI. We analyzed secondary outcomes using the PROC LOGISTIC procedure in SAS (9.4); we performed all other analyses in R v3.5.2 (Cary, NC).
RESULTS
Patient Characteristics
A total of 3,464 individuals met our definition for critically injured patients. The primary reason for exclusion was lack of a trauma diagnosis from the trauma registry. Baseline patient and visit characteristics are presented in Table 1. Mean age was 53 years. Patients were 65% male, 68% non-Hispanic White, 8% non-Hispanic Black, and 16% Hispanic. Blunt trauma mechanism was present in 66% of cases, 73% arrived by emergency medical services, and the median APACHE II score was 8, which corresponds to a predicted ICU mortality of 8%.
TABLE 1.
Characteristics and Outcomes of Colorado ICU Admissions for Major Trauma 2015–2018
| Characteristics | All, n = 3,464 | Died, n = 264 (7.6%) | Survived, n = 3,200 | p |
|---|---|---|---|---|
| Demographics, n (%) | ||||
| Age, yr, mean (sd) | 52.6 (21.0) | 56.9 (20.7) | 52.2 (21.0) | < 0.001 |
| Male gender | 2,263 (65) | 178 (67) | 2,085 (65) | 0.503 |
| Race/ethnicity | ||||
| Non-Hispanic White | 2,346 (68) | 157 (59) | 2,189 (68) | < 0.001 |
| Hispanic | 547 (16) | 46 (17) | 501 (16) | |
| Non-Hispanic Black | 280 (8) | 14 (5) | 266 (8) | |
| Other | 291 (8) | 47 (18) | 244 (8) | |
| Insurance status | ||||
| Private | 1,155 (33) | 64 (24) | 1,091 (34) | < 0.001 |
| Medicare | 1,122 (32) | 113 (43) | 1,009 (32) | |
| Medicaid | 1,004 (29) | 57 (22) | 947 (30) | |
| Other | 31 (1) | 3 (1) | 28 (1) | |
| Missing | 152 (4) | 27 (10) | 125 (4) | |
| Medical history | ||||
| Comorbidities | ||||
| Any cardiopulmonary, n (%) | 669 (19) | 75 (28) | 594 (19) | < 0.001 |
| Number of other, median (IQR) | 3 (1-4) | 4 (2-5) | 3 (1-5) | |
| Tobacco use, n (%) | ||||
| Current or former smoker | 1,489 (43) | 71 (27) | 1,418 (44) | < 0.001 |
| Never smoker | 1,226 (35) | 47 (18) | 1,179 (37) | |
| Not reported/missing | 749 (22) | 146 (55) | 603 (19) | |
| Alcohol use, n (%) | ||||
| Yes | 1,475 (43) | 58 (22) | 1,417 (44) | < 0.001 |
| No | 1,216 (35) | 66 (25) | 1,150 (36) | |
| Not reported/missing | 773 (22) | 140 (53) | 633 (20) | |
| Other substance use, n (%) | ||||
| Yes | 642 (19) | 16 (6) | 626 (20) | < 0.001 |
| No | 1,921 (55) | 93 (35) | 1,828 (57) | |
| Not reported/missing | 901 (26) | 155 (59) | 746 (23) | |
| Clinical | ||||
| Emergency medical services arrival, n (%) | 2,525 (73) | 221 (84) | 2,304 (72) | < 0.001 |
| Mechanism of injury, n (%) | ||||
| Fall | 1,235 (36) | 102 (39) | 1,133 (35) | 0.012 |
| Motor vehicle | 1,069 (31) | 67 (25) | 1,002 (31) | |
| Penetrating | 258 (7) | 31 (12) | 227 (7) | |
| Other | 902 (26) | 64 (24) | 838 (26) | |
| Ever mechanically ventilated, n (%) | 1,421 (41) | 249 (94) | 1,172 (37) | < 0.001 |
| Acute Physiology and Chronic Health Evaluation II score, median (IQR) | 8 (5-14) | 22 (15-27) | 7 (5-12) | < 0.001 |
| Length of stay, d, median (IQR) | 6 (3-11) | 4 (1-9) | 6 (3-12) | < 0.001 |
IQR = interquartile range.
The percentage of time spent by patients in each Fio2 and Spo2 category during the first 3 days and days 4–7 of hospitalization are presented in Figure 1. Of the 226,057 patient-hours recorded, 186,902 (46%) were spent with hyperoxia, 212,360 (52%) with normoxia, 2,499 (1%) with mild hypoxia, and 3,921 (1%) with hypoxia.
Figure 1.
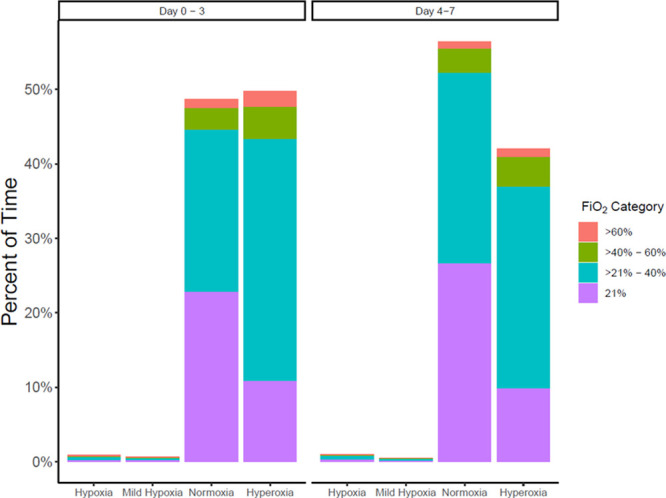
Percentage of time in each Spo2 and Fio2 category in the first 3 d and days 4–7 of hospitalization. Hypoxia (oxygen saturation [Spo2] < 88%), mild hypoxia (Spo2 88–89%), normoxia (Spo2 90–96%), hyperoxia (Spo2 > 96%).
Primary Outcome
The primary outcome (in-hospital mortality) occurred in 264 patients (7.6%). One-thousand four-hundred twenty-one patients (41%) required mechanical ventilation outside of the operating room at some point during their index admission. The median hospital length of stay was 6 days (IQR, 3–12 d) among survivors.
As Fio2 increased, risk for mortality increased across all Spo2 levels. The oxygenation variables jointly were significantly associated with mortality according to likelihood ratio testing (p < 0.001). Graphical and tabular summaries of model results were produced to interpret the direction of these effects. The adjusted risk scores for mortality at all Fio2 and Spo2 categories are presented as a heatmap in Figure 2. The reference is an individual with the mean value of each covariate. We calculated risk scores from Cox models adjusted for race, age, gender, APACHE II score, smoking status, cardiopulmonary comorbidities, number of noncardiopulmonary comorbidities, and MOI. During hyperoxia (Spo2>96%), the risk for mortality increased with greater Fio2.
Figure 2.
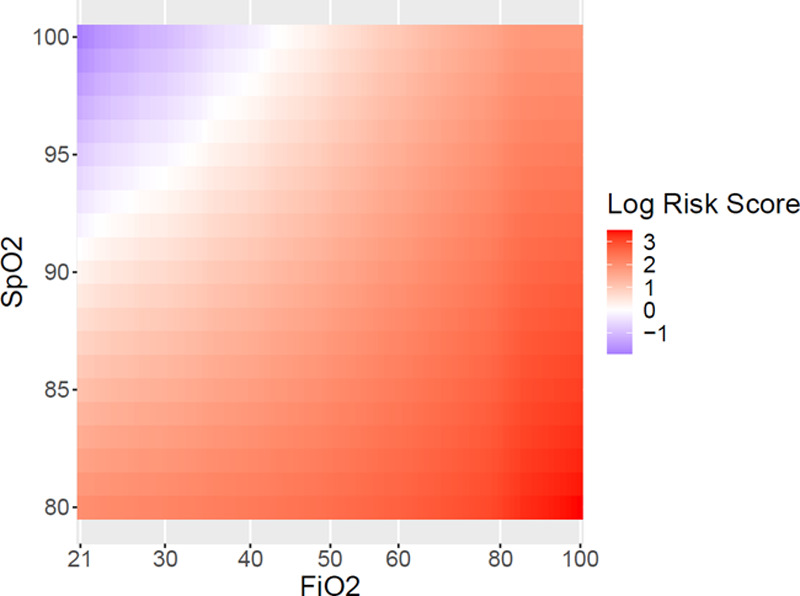
Heatmap of risk scores for mortality at each oxygen saturation (Spo2) and Fio2 level. For each Spo2 level, greater Fio2 increases risk of mortality.
Adjusted and unadjusted risk scores for mortality at specific Spo2 and Fio2 values are presented in Supplemental Table 2 (http://links.lww.com/CCX/A608) and Supplemental Table 3 (http://links.lww.com/CCX/A609), respectively. During hyperoxia, the adjusted risk of mortality was higher for greater Fio2. That is, at a given Spo2, higher Fio2 increased the risk for mortality, and this effect was more pronounced at higher Spo2 levels. Adjusted hazard ratios for mortality for all variables used in the Cox models are presented in Table 2.
TABLE 2.
Hazard Ratios for Mortality of All Covariates Used in the Cox Models
| Covariates | Hazard Ratio (95% CI) |
|---|---|
| Continuous | |
| Age | 1.03 (1.02–1.03) |
| Acute Physiology and Chronic Health Evaluation II Score | 1.08 (1.06–1.09) |
| Number of Noncardiopulmonary comorbidities | 0.92 (0.86–0.98) |
| Categorical | |
| Other race/Ethnicity | 1.23 (0.93–1.62) |
| Female | 1.13 (0.86–1.50) |
| Never smoker | 0.77 (0.51–1.14) |
| Unknown smoking status | 1.87 (1.35–2.60) |
| Cardiopulmonary comorbidities present | 1.00 (0.74–1.35) |
| Nonblunt mechanism of injury | 0.97 (0.73–1.30) |
Hazard ratio (HR) for age accounts for every 1 yr increase. HR for Acute Physiology and Chronic Health Evaluation II score accounts for every one-point increase.
Secondary Outcomes
Median VFDs to day 28 was 28 days (IQR, 25–28 d). Median HFDs to day 90 was 83 days (IQR, 75–87 d). The odds ratios (ORs) for each category of VFDs/HFDs relative to death estimated from multinomial regression models are displayed in Figure 3. Our analysis showed generally that more time on room air and less time hyperoxic were associated with favorable outcomes. An increased amount of time on room air (Fio2 21%) was associated with an increased in the odds of more VFDs and HFDs. For each 1% point increase in time on room air, the odds of being in the 28 VFDs group versus death increased (OR, 1.06; 95% CI, 1.05–1.07) For each 1% point increase in time on room air, the odds of being in the 84–90 HFDs group also increased (OR, 1.04; 95% CI, 1.03–1.05). Conversely, increased time in hyperoxia (Spo2 > 96%) was associated with decreased odds of greater VFDs and HFDs. For each 1% point increase in time in hyperoxia, the odds of never requiring mechanical ventilation versus death decreased (OR, 0.99; 95% CI, 0.98–0.99). Similarly, for each 1% point increase in time in hyperoxia, the odds of being in the 84–90 HFDs group was also decreased (OR, 0.99; 95% CI, 0.98–1.00).
Figure 3.
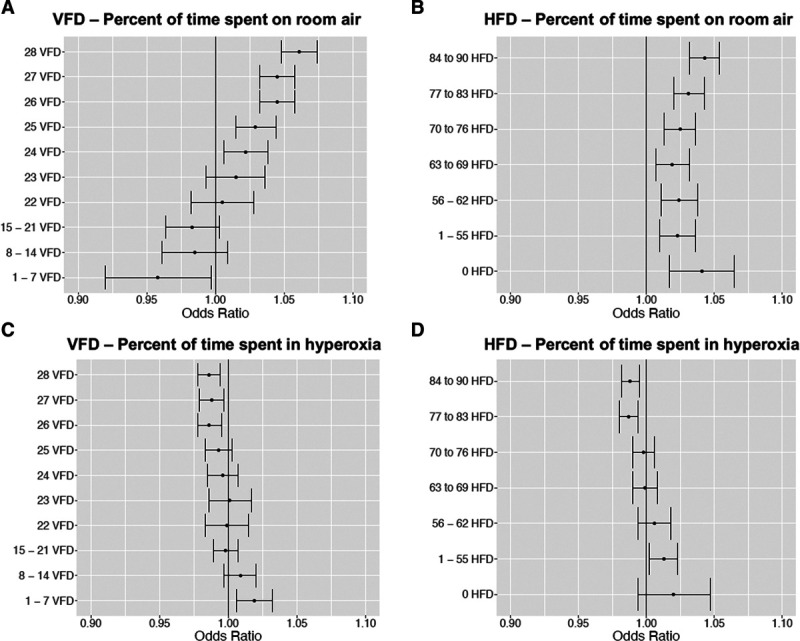
Adjusted odds ratios (ORs) and 95% CIs for ventilator-free days (VFDs) and hospital-free days (HFDs); all odds ratios are relative to death. All models were adjusted for age, race/ethnicity, sex, smoking status, Acute Physiology and Chronic Health Evaluation II score, presence of cardiopulmonary comorbidities, number of other comorbidities, and mechanism of injury. A, ORs for percent of time on room air at each VFDs group. B. ORs for percent of time on room air at each HFDs group. C, ORs for percent of time in hyperoxia at each VFDs group. D, ORs for percent of time in hyperoxia at each HFDs group. *By definition, people with zero VFDs will spend an extremely low percentage of time on room air; as a result, this odds ratio (0 VFDs relative to death) was excluded from the figure due to large CIs (OR, 0.79; 95% CI, 0.66–0.95).
DISCUSSION
In this large multicenter cohort of critically injured patients, hyperoxia was present on average during nearly half of the time during the first 7 days of hospitalization. During hyperoxia, higher oxygen administration was associated with a greater risk of mortality.
The mortality risk associated with increasing oxygen administration was greatest at higher Spo2 levels (Fig. 2). The lowest mortality risk for each Spo2 level was attained at 21% Fio2 or room air. Many hyperoxic patients (Spo2 > 96%) in our study continued to be ventilated with Fio2 greater than room air. One explanation involves the standardized order sets dictating respiratory therapist practice. During the study period, these order sets did not allow for titration of Fio2 below 40% for mechanically ventilated patients. Since critically injured patients in this study spent nearly half of their time in hyperoxia, this may represent an inappropriate use of oxygen and a potential “modifiable” area for improvement. These patients should be weaned to room air as quickly as possible to minimize the risk for mortality associated with hyperoxia. However, the clinical indication for hyperoxia was not investigated for each instance, and it is possible these choices were justified.
A small but significant protective effect for mortality was associated with a greater number of noncardiopulmonary comorbidities (Table 2). This appears counter-intuitive. However, the effect is likely offset by the trend toward increased mortality for patients with a greater number of cardiopulmonary comorbidities.
Overall, more time in hyperoxia was associated with fewer VFDs and HFDs. This association was most pronounced at the extremes of duration of mechanical ventilation and length of stay.
This cohort study distinguishes itself from other retrospective hyperoxia studies by virtue of three key methodological innovations. First, we are not aware of prior studies which assess oxygenation continuously throughout hospitalization. Previous studies investigating hyperoxia and in trauma patients (28–31) and critically ill medical patients (3, 5–8, 32–41) recorded either the highest Pao2 within the first 24 hours or a mean Pao2. The definition of “hyperoxia” in these previous trials also ranged from a Pao2 greater than 100 mm Hg to a Pao2 greater than 300 mm Hg. Therefore, this is the first study to investigate oxygenation for the entire duration of hospitalization with a very high frequency of discrete measurements. Second, due to our treatment of oxygenation as a continuous variable, more sophisticated models were required when compared with previous hyperoxia studies. Nonlinear terms were included in a Cox regression model to estimate the association between oxygenation variables and mortality risk, as detailed in the Methods section. This level of sophistication is likely why we are the first group, to our knowledge, to apply such a method. Third, many of the previous studies examining the association between hyperoxia and mortality in trauma patients focused almost exclusively on TBI (29, 30, 42–54). One of the strengths of our study is the inclusion of all trauma patients: blunt, penetrating, or otherwise. This increases the external generalizability of our results due to the broad scope of included patients.
Given the association between hyperoxia and supplemental oxygen delivery with in-hospital mortality for critically injured patients, it is time to begin rethinking our clinical practice. As many as 84% of critically ill patients (both medical and trauma) are exposed to excess levels of oxygen (6, 13). The recent ICU-randomized trial comparing two approaches to oxygen therapy randomized controlled trial found no significant difference in VFDs between critically ill patients who received conservative versus usual oxygen therapy (55). However, a recent meta-analysis of critically ill patients demonstrated that supplemental oxygen to achieve an Spo2 greater than 96% increased in-hospital mortality without improving other “patient-important” outcomes (13). In our study, additional oxygen administration was associated with increased mortality for already hyperoxic patients. Therefore, hyperoxia appears to not just increase mortality but does so in a dose-dependent manner. This clinically important association, if validated by rigorous clinical trials, could be practice changing. Such clinical trials are already underway (NCT04534959). Just as clinicians administer judicious volumes of IV fluids to their critically injured patients, less aggressive oxygen support also has potential to reduce morbidity and mortality.
Avoidance of hyperoxia could also improve resource utilization. Oxygen is a limited resource with significant obstacles in transporting to austere setting. Substantial resources, space, and weight must be dedicated to oxygen during such missions. Further, the coronavirus disease 2019 pandemic has exposed flaws in many healthcare systems around the world (56–58). Although we investigated exclusively civilian trauma patients, our results are unlikely to be different for active-duty service members. Therefore, the association between hyperoxia and mortality also has the benefit of decreasing the logistic burden of oxygen procurement in military operations.
The results of this study should be interpreted in the context of some limitations. This study is retrospective in nature. The primary outcome (in-hospital mortality) was relatively uncommon, occurring in just 7.6% of the sample. The large size of our cohort (3,464 patients) partially mitigates this issue. Illness Severity Score was not available for all patients; therefore, APACHE II score was used a surrogate. It is also possible the proportionality of hazard assumption used for the Cox regression is not valid for this study. We employed several assumptions in our cohort, including time of death and duration of mechanical ventilation. In addition, we assumed all patients discharged on a ventilator remain on a ventilator until day 28, and all patients discharged off ventilation, remained ventilator free until day 28 (24). Further, we did not attempt to find an ideal oxygenation target for critically injured patients. Our definitions for hypoxia, normoxia, and hyperoxia are based on previous studies and a Delphi consensus (20). It is possible that an Spo2 of 90–96% does not necessarily represent normoxia in trauma. Perhaps, oxygenation targets for critically injured patients should fall into a different range. Prospective clinical trials are needed to further elucidate ideal oxygenation targets. A phase 3, multicenter randomized controlled trial (NCT04534959) will investigate the question of causality between hyperoxia and mortality in critically injured patients. Finally, oxygen delivery and target levels may vary based on altitude; all three sites are more than 5,000 feet above sea level (5,003 feet in Fort Collins, CO, 5,403 feet in Aurora, CO, and 6,035 feet in Colorado Springs, CO). This correlates to partial pressures of atmospheric oxygen ranging from 128 mm Hg to 133 mm Hg, compared with 160 mm Hg at sea level (59). Therefore, extrapolation of our findings to sea level requires further research.
CONCLUSIONS
In this large multicenter cohort of critically injured civilian patients, hyperoxia was present nearly half of the time during the first 7 days of hospitalization. During hyperoxia, higher oxygen administration was associated with a greater risk of mortality. Prospective interventional studies are required to determine the causal association between hyperoxia and clinical outcomes and optimal target oxygen concentrations in critically injured patients.
ACKNOWLEDGEMENTS
We thank Caroline M. Ledbetter, MPH, and Yang Wang, PhD, for their assistance with the preliminary data analysis for this study.
Supplementary Material
Footnotes
This article expresses the authors’ opinions and does not reflect the policy or opinions of the Department of the Army, Department of the Air Force, Department of Defense, or U.S. Government.
Drs. Douin, Anderson, Schauer, and Ginde contributed to study concept and design. Drs. Douin, Anderson, and Ginde contributed to acquisition of data. Drs. Rice and Jackson contributed to statistical analysis. All authors contributed to interpretation of data and critical revision of the article for important intellectual content. Drs. Douin and Anderson contributed to drafting of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the Department of Defense/Special Operations Command (W81XWH-17-C-0241).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.McMullan J, Rodriquez D, Hart KW, et al. Prevalence of prehospital hypoxemia and oxygen use in trauma patients. Mil Med. 2013; 178:1121–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetterslev J, Meyhoff CS, Jørgensen LN, et al. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. Cochrane Database Syst Rev. 2015; 2015:CD008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilgannon JH, Jones AE, Shapiro NI, et al. ; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010; 303:2165–2171 [DOI] [PubMed] [Google Scholar]
- 4.Smit B, Smulders YM, de Waard MC, et al. Moderate hyperoxic versus near-physiological oxygen targets during and after coronary artery bypass surgery: A randomised controlled trial. Crit Care. 2016; 20:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panwar R, Hardie M, Bellomo R, et al. ; CLOSE Study Investigators; ANZICS Clinical Trials Group. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016; 193:43–51 [DOI] [PubMed] [Google Scholar]
- 6.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The oxygen-ICU randomized clinical trial. JAMA. 2016; 316:1583–1589 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Eastwood GM, Glassford NJ, et al. Conservative oxygen therapy in mechanically ventilated patients: A pilot before-and-after trial. Crit Care Med. 2014; 42:1414–1422 [DOI] [PubMed] [Google Scholar]
- 8.Johnson NJ, Dodampahala K, Rosselot B, et al. The association between arterial oxygen tension and neurological outcome after cardiac arrest. Ther Hypothermia Temp Manag. 2017; 7:36–41 [DOI] [PubMed] [Google Scholar]
- 9.Damiani E, Adrario E, Girardis M, et al. Arterial hyperoxia and mortality in critically ill patients: A systematic review and meta-analysis. Crit Care. 2014; 18:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert-Kawai ET, Mitchell K, Martin D, et al. Permissive hypoxaemia versus normoxaemia for mechanically ventilated critically ill patients. Cochrane Database Syst Rev. 2014; 2014:CD009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association between arterial hyperoxia and outcome in subsets of critical illness: A systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med. 2015; 43:1508–1519 [DOI] [PubMed] [Google Scholar]
- 12.Wang CH, Chang WT, Huang CH, et al. The effect of hyperoxia on survival following adult cardiac arrest: A systematic review and meta-analysis of observational studies. Resuscitation. 2014; 85:1142–1148 [DOI] [PubMed] [Google Scholar]
- 13.Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet. 2018; 391:1693–1705 [DOI] [PubMed] [Google Scholar]
- 14.Douin DJ, Schauer SG, Anderson EL, et al. Systematic review of oxygenation and clinical outcomes to inform oxygen targets in critically ill trauma patients. J Trauma Acute Care Surg. 2019; 87:961–977 [DOI] [PubMed] [Google Scholar]
- 15.Leydesdorff L, Comins JA, Sorensen AA, et al. Cited references and Medical Subject Headings (MeSH) as two different knowledge representations: Clustering and mappings at the paper level. Scientometrics. 2016; 109:2077–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Reilly Nugent A, Kelly PT, Stanton J, et al. Measurement of oxygen concentration delivered via nasal cannulae by tracheal sampling. Respirology. 2014; 19:538–543 [DOI] [PubMed] [Google Scholar]
- 17.Wagstaff TA, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anaesthesia. 2007; 62:492–503 [DOI] [PubMed] [Google Scholar]
- 18.Waldau T, Larsen VH, Bonde J. Evaluation of five oxygen delivery devices in spontaneously breathing subjects by oxygraphy. Anaesthesia. 1998; 53:256–263 [DOI] [PubMed] [Google Scholar]
- 19.Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005; 50:604–609 [PubMed] [Google Scholar]
- 20.Ginde A. Targeted normoxia to conserve oxygen and improve clinical outcomes in combat injured special operations forces. Defense Technical Information Center. 2020.Available at: https://apps.dtic.mil/sti/citations/AD1101367 [Google Scholar]
- 21.Capuzzo M, Valpondi V, Sgarbi A, et al. Validation of severity scoring systems SAPS II and APACHE II in a single-center population. Intensive Care Med. 2000; 26:1779–1785 [DOI] [PubMed] [Google Scholar]
- 22.Databases H. Healthcare cost and utilization project (HCUP). Agency for Heatlhcare Research and Quality, Rockville, MD, 2009. Available at: www.hcup-us.ahrq.gov/databases.jsp [PubMed] [Google Scholar]
- 23.World Health Organization: ICD-10: International. Statistical Classification of Diseases and Related Health Problems: Tenth Revision. Geneva, Switzerland: World Health Organization (WHO), 2004; 2nd ed. Available at: https://apps.who.int/iris/handle/10665/42980 [Google Scholar]
- 24.Yehya N, Harhay MO, Curley MAQ, et al. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019; 200:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenfeld DA, Bernard GR; ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002; 30:1772–1777 [DOI] [PubMed] [Google Scholar]
- 26.Krause M, Douin DJ, Kim KK, et al. Characteristics and outcomes of mechanically ventilated COVID-19 patients - an observational cohort study. J Intensive Care. 2020; 36:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krause M, Douin DJ, Tran TT, et al. Association between procalcitonin levels and duration of mechanical ventilation in COVID-19 patients. PLoS One. 2020; 15:e0239174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DesPrez K, McNeil JB, Wang C, et al. Oxygenation saturation index predicts clinical outcomes in ARDS. Chest. 2017; 16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner M, Stein D, Hu P, et al. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012; 147:1042–1046 [DOI] [PubMed] [Google Scholar]
- 30.Davis DP, Meade W, Sise MJ, et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009; 26:2217–2223 [DOI] [PubMed] [Google Scholar]
- 31.Stockinger ZT, Mcswain NE, Jr. Prehospital supplemental oxygen in trauma patients: Its efficacy and implications for military medical care. Mil Med. 2004; 169:609–612 [DOI] [PubMed] [Google Scholar]
- 32.Schernthaner C, Wernly B, Lichtenauer M, et al. High peak PaO2 values are associated with adverse outcome in critically ill patients treated with non-invasive ventilation for acute cardogenic pulmonary edema and pneumonia. Panminerva Medica. 2017; 07:07. [DOI] [PubMed] [Google Scholar]
- 33.Staehr-Rye AK, Meyhoff CS, Scheffenbichler FT, et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth. 2017; 119:140–149 [DOI] [PubMed] [Google Scholar]
- 34.Wang HE, Prince DK, Drennan IR, et al. ; Resuscitation Outcomes Consortium (ROC) Investigators. Post-resuscitation arterial oxygen and carbon dioxide and outcomes after out-of-hospital cardiac arrest. Resuscitation. 2017; 120:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmer J, Scutella M, Pullalarevu R, et al. ; Pittsburgh Post-Cardiac Arrest Service (PCAS). The association between hyperoxia and patient outcomes after cardiac arrest: Analysis of a high-resolution database. Intensive Care Med. 2015; 41:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rincon F, Vibbert M, Urtecho J, et al. Hyperoxia is associated with higher case-fatality in ventilated patients with intra-cerebral hemorrhage. Crit Care Shock. 2015; 18:61–71 [Google Scholar]
- 37.Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: A multicenter cohort study. Crit Care Med. 2014; 42:387–396 [DOI] [PubMed] [Google Scholar]
- 38.Wang CH, Huang CH, Chang WT, et al. Association between early arterial blood gas tensions and neurological outcome in adult patients following in-hospital cardiac arrest. Resuscitation. 2015; 89:1–7 [DOI] [PubMed] [Google Scholar]
- 39.Janz DR, Hollenbeck RD, Pollock JS, et al. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012; 40:3135–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilgannon JH, Jones AE, Parrillo JE, et al. ; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011; 123:2717–2722 [DOI] [PubMed] [Google Scholar]
- 41.de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008; 12:R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Costa LGV, Carmona MJC, Malbouisson LM, et al. Independent early predictors of mortality in polytrauma patients: A prospective, observational, longitudinal study. Clinics (Sao Paulo). 2017; 72:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taher A, Pilehvari Z, Poorolajal J, et al. Effects of normobaric hyperoxia in traumatic brain injury: A randomized controlled clinical trial. Trauma Mon. 2016; 21:e26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascia L, Zavala E, Bosma K, et al. ; Brain IT group. High tidal volume is associated with the development of acute lung injury after severe brain injury: An international observational study. Crit Care Med. 2007; 35:1815–1820 [DOI] [PubMed] [Google Scholar]
- 45.Chi JH, Knudson MM, Vassar MJ, et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: A prospective multicenter study. J Trauma. 2006; 61:1134–1141 [DOI] [PubMed] [Google Scholar]
- 46.Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004; 57:1–8 [DOI] [PubMed] [Google Scholar]
- 47.Tolias CM, Reinert M, Seiler R, et al. Normobaric hyperoxia–induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: A prospective historical cohort-matched study. J Neurosurg. 2004; 101:435–444 [DOI] [PubMed] [Google Scholar]
- 48.Manley G, Knudson MM, Morabito D, et al. Hypotension, hypoxia, and head injury: Frequency, duration, and consequences. Arch Surg. 2001; 136:1118–1123 [DOI] [PubMed] [Google Scholar]
- 49.Gentleman D. Causes and effects of systemic complications among severely head injured patients transferred to a neurosurgical unit. Int Surg. 1992; 77:297–302 [PubMed] [Google Scholar]
- 50.Fujita M, Oda Y, Yamashita S, et al. Early-stage hyperoxia is associated with favorable neurological outcomes and survival after severe traumatic brain injury: A post-hoc analysis of the brain hypothermia study. J Neurotrauma. 2017; 34:1565–1570 [DOI] [PubMed] [Google Scholar]
- 51.Russell DW, Janz DR, Emerson WL, et al. Early exposure to hyperoxia and mortality in critically ill patients with severe traumatic injuries. BMC Pulm Med. 2017; 17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raj R, Bendel S, Reinikainen M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013; 17:R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones PA, Andrews PJ, Midgley S, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol. 1994; 6:4–14 [PubMed] [Google Scholar]
- 54.Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996; 40:764–767 [DOI] [PubMed] [Google Scholar]
- 55.Mackle D, Bellomo R, Bailey M, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. New Engl J Med. 2019; 382:989–998 [DOI] [PubMed] [Google Scholar]
- 56.Calligaro GL, Lalla U, Audley G, et al. The utility of high-flow nasal oxygen for severe COVID-19 pneumonia in a resource-constrained setting: A multi-centre prospective observational study. EClinicalMedicine. 2020; 28:100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aziz S, Arabi YM, Alhazzani W, et al. Managing ICU surge during the COVID-19 crisis: Rapid guidelines. Intensive Care Med. 2020; 46:1303–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birkmeyer JD, Barnato A, Birkmeyer N, et al. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood). 2020; 39:2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortiz-Prado E, Dunn JF, Vasconez J, et al. Partial pressure of oxygen in the human body: A general review. Am J Blood Res. 2019; 9:1–14 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


