Figure 5. Endothelialization of decelluiarized vessels using XF-hiPSC-ECs.
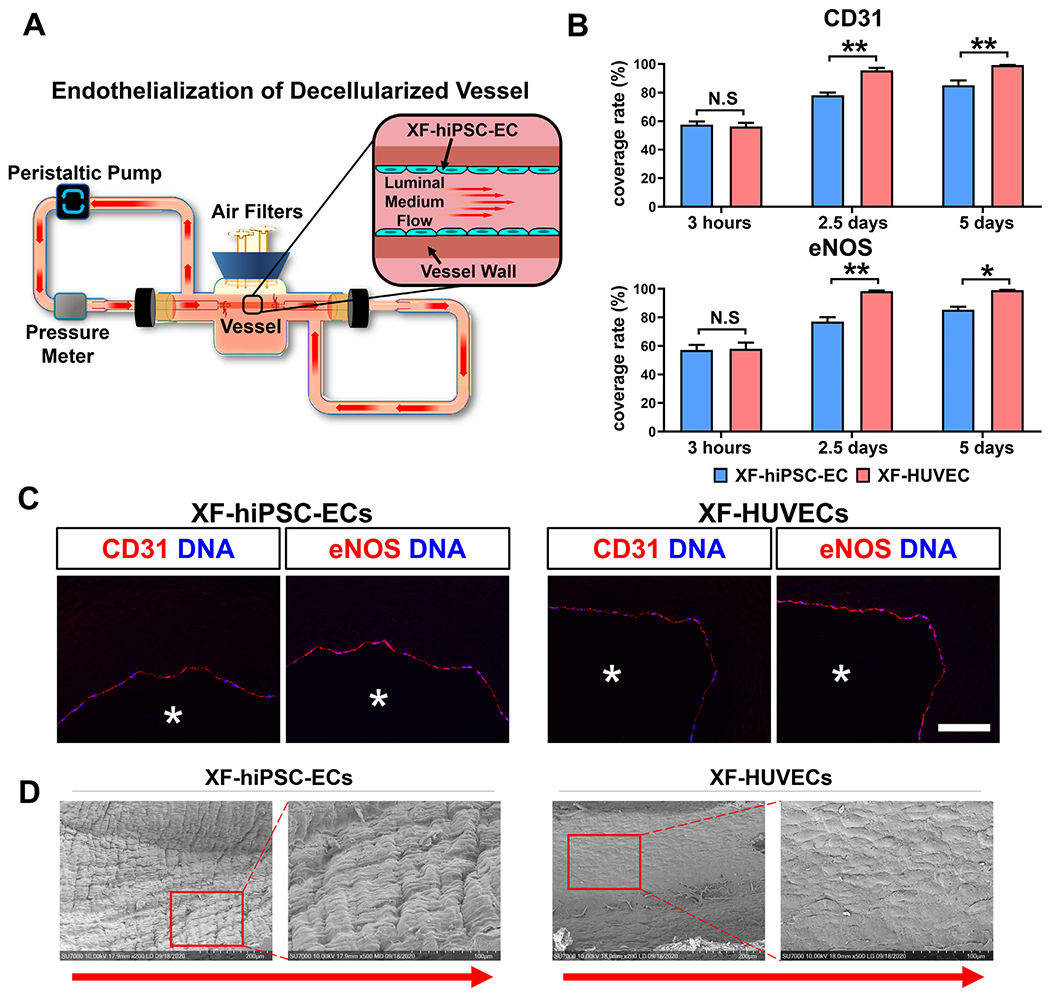
(A) Schematic illustration of the dynamic bioreactor system for endothelializing deceiluiarized human vessels using XF-hiPSC-ECs. (B) Percentage of the luminal surface of decelluiarized vessels covered by the XF-hiPSC-ECs or XF-HUVECs 3 hours (prior to adding shear stress), 2.5 days and 5 days after cell seeding based on the staining in Fig. S11. Quantification was performed based on three representative sections in each endothelialized vessel, and three vessels were independently endothelialized using each cell type (Two-way ANOVA showed that there was significant interaction between the time point and cell type/culture conditions [CD31: p<0.01; eNOS: p<0.05]. Mean values and S.E.M indicated by the error bars are shown; n=3; *: p<0.05; **: p<0.01; N.S: not significant). (C) Immunofluorescence staining of EC markers (CD31 and eNOS) of decelluiarized vessels after endotheiialization using XF-hiPSC-ECs or XF-HUVECs for 5 days (original magnification: 20x). DNA (nuclear) was counterstained by DAPI in immunostaining. Asterisk indicates the vessel lumen. Scale bar: 100 μm. (D) Representative images of luminal surface of decellularized human vessel endothelialized with XF-hiPSC-ECs or XF-HUVECs for 5 days via scanning electron microscopy. The right panels for each cell type (magnification 500X) indicate the magnified areas within the red frames in left images (magnification: 200X). Direction of arrows indicates the direction of luminal medium flow.
