FIGURE 1.
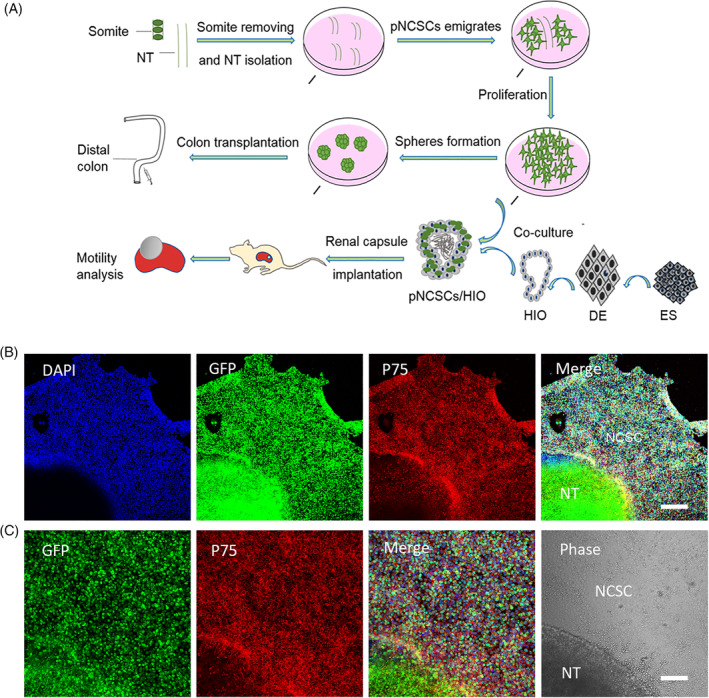
The experimental workflow for manipulating premigratory neural crest stem cells (pNCSCs) and primary characteristics of pNCSCs in culture. A, Schematic diagram of workflow for isolation of the pNCSCs, cell proliferation, subrenal capsule implantation, and transplantation. The pieces of mouse neural tubes (NT) at E9.0 are placed in the proliferation medium. The emigrated cells from NT are manually isolated and cultured as P0. These cells are proliferated to P3 or P4 before transplantation or cocultured with human intestinal organoids (HIO). With respect to the route of transplantation, spheres formed from pNCSCs are transplanted into the distal colon of mice to check the capacity to regenerate the enteric nerve. The other route is used for checking if the intestinal motility could be mediated by the tissue‐engineered intestine's (TEI) pNCSCs‐derived neurons. Human embryonic stem cells (hESCs) are first induced into the definitive endoderm (DE), and DE is further induced into the HIO. For TEI maturation, HIO and HIO + pNCSCs are transplanted into the kidney capsule of BALB/c nude mice to mature. The mature TEI is further analyzed for intestine motility. B, The immunostaining of P75, DAPI, EGFP, and the merged images of the cells migrated from NT in the first batch in sequence. The cells displaying positive P75 immunostaining are presumably NCSCs. C, Higher magnification of the inset in (B) about EGFP, P75 staining, merged image of EGFP, DAPI and P75 staining, and phase image in the sequence. The supposed pNCSCs are shown in the phase image. Scale bars = 200 μm (B), 100 μm (C)
