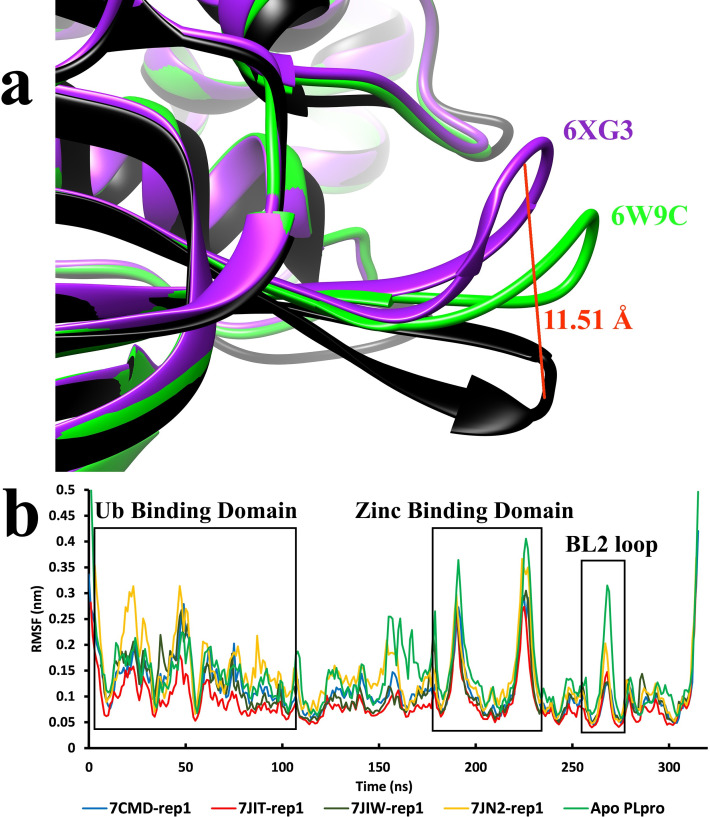
Fig 6. The flexibility of the BL2 loop.
(A) the crystallographic structures of the Plpro are superimposed to compare the flexibility of the BL2 loop and the degree of its opening mechanism. The fully open structure of the BL2 (Black), which was achieved from the unbinding pathways and in the presence of the inhibitor, showed that the distance between the Cα atoms of the Tyr268 on the tip of the BL2 loop in each structure reaches 11.5 Å. (B) the RMSF values of the residues of the Plpro enzyme during the unbinding pathways of the inhibitors and in the apo form of the enzyme, with respect to the important regions of the enzyme.