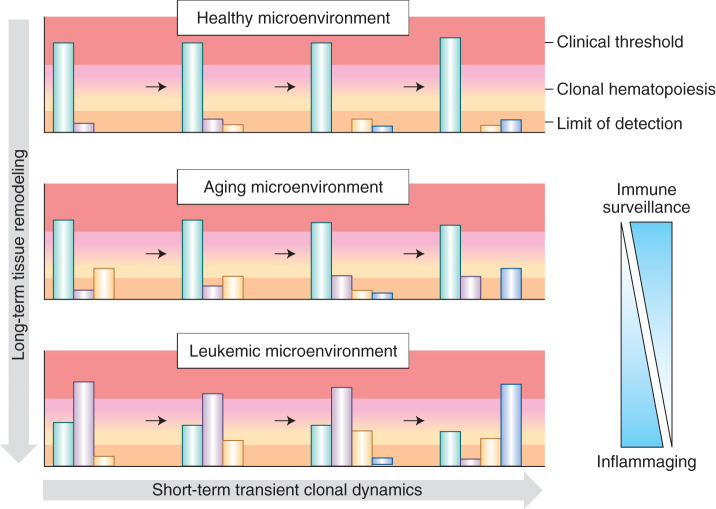
Figure 3.
Dynamic selective pressures imposed by the microenvironment with aging and disease pathogenesis result in differential expansion of clones. In early life, immune surveillance results in purifying selection and effective removal of (pre-)malignant clones, thereby preventing their expansion and, in turn, detection. Immune clearance of malignant cells evidently declines with age in the phenomenon known as immunosenescence, in part, providing an explanation for the greater incidences of myeloid malignancies in the elderly (114). For a number of reasons, including thymic involution and increasing ratios of regulatory T cells to cytotoxic T cells, (pre-)malignant cells expressing neoantigens that would otherwise be eliminated by immune surveillance are permitted to persist and expand in old age (114,115,116,117,118). Later in life, the increasing selective pressure of inflammaging can lead to clonal expansions and clonal hematopoiesis. Finally, in leukemia, malignant clones are selected for and completely consume the bone marrow, leading to symptomatic consequences. The upper range depicted in red represents the threshold at which the buffering by remaining healthy hematopoietic clones is no longer sufficient to achieve a canalized healthy phenotype and clinical symptoms emerge. The lower range depicted in dark peach represents the sequencing limit of detection. The green represents phenotypically wild-type clones, while the other colors represent (pre-)malignant clones with pathogenic mutation(s). In the aging microenvironment, the wild-type clones retain enough of a selective advantage to sustain hematopoiesis and prevent any clinical consequences; however, upon progression to leukemia, the bone marrow microenvironment has been remodeled to favor mutated clones over wild-type (green). The wild-type clones can no longer sustain healthy hematopoiesis, and the patient will present with symptoms of leukemia.