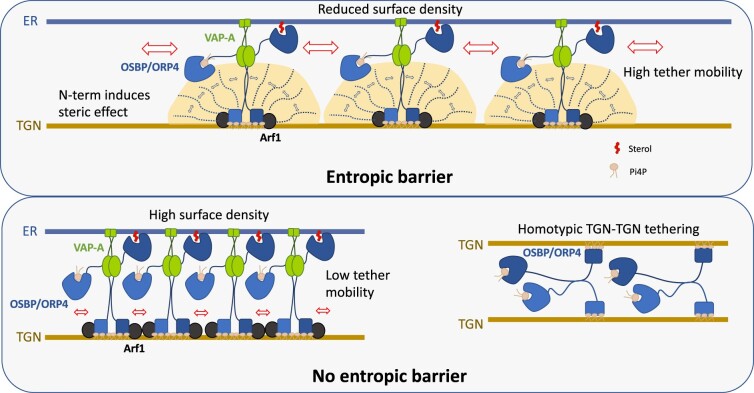
Figure 2.
An entropic barrier controls membrane tethering geometry. Top: OSBP/ORP4 (blue dimers) transports cholesterol from the ER to the trans-Golgi network (TGN) and back transfer PI4P in exchange. They interact via their PH domain, with PI4P and the small G protein Arf1 (black) on the TGN membrane and with the ER-resident protein VAP-A (Green) via their FFAT motif. Both OSBP and ORP4 present a long N-terminal unstructured region (N-term), which through steric effect controls OSBP/ORP4 density at MCS by limiting the surface density of the downstream PH domain. This in turn facilitates protein diffusion within MCS and protein recycling between MCS. The N-term region also controls the geometry of ER–TGN MCSs and protein orientation by favoring heterotypic membrane tethering (TGN–ER) between VAP-containing and PI4P-containing membranes. Bottom: In the absence of the N-term region, protein density is higher and mobility reduced (left). Homotypic configuration (TGN–TGN), where two PI4P-rich TGN membranes are tethered by the two PH domains of OSBP/ORP4, is now favored. Adapted from Jamecna et al. (2019). Arf1: adenosine diphosphate-ribosylation factor; FFAT: two phenylalanines in an acidic tract; PH: pleckstrin homology; TGN: trans-Golgi network; VAP-A: vesicle-associated membrane protein-associated protein A.