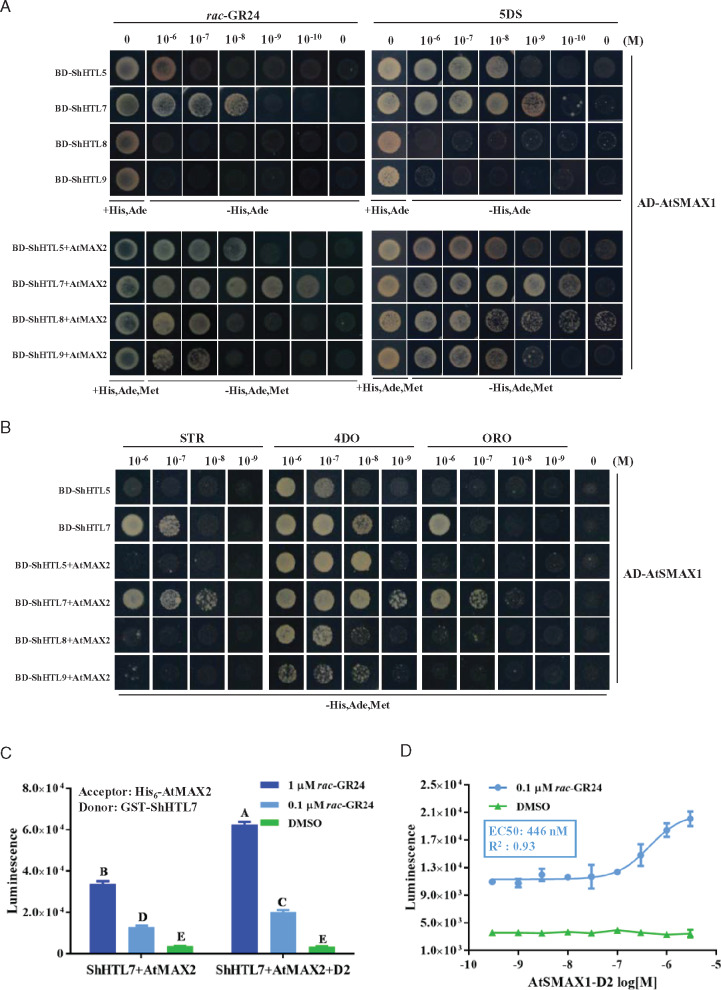
Figure 3.
AtMAX2 and AtSMAX1 enhance the SL-induced binding of each other to ShHTL. (A) Y2H and Y3H assay of interactions between AtSMAX1 and ShHTL5, ShHTL7, ShHTL8, and ShHTL9 in the absence or presence of AtMAX2 were detected in the presence of 10−6 to 10−10 M rac-GR24 (left panel) and 5DS (right panel). AtSMAX1 was fused with the Gal4 AD-SMAX1 and ShHTLs were fused with Gal4 DNA BD as bait (BD-ShHTLs). AtMAX2 fused with AtASK1 at its N-terminus was co-expressed with ShHTL5, ShHTL7, ShHTL8, and ShHTL9. (B) Y2H assay of the interaction of AD-AtSMAX1 with ShHTL5 and ShHTL7 and interaction of AD-AtSMAX1 with ShHTL5, ShHTL7, ShHTL8, and ShHTL9 in the presence of AtMAX2 were assessed in response to 4DO, STR, and ORO in the concentration range 10−6 to 10−9 M. Interactions were detected in the control medium and the selective medium SD/-Leu/-Trp/-His/-Ade (-His, Ade) for Y2H and SD/-Leu/-Trp/-His/-Ade/-Met (-His, Ade, Met) for Y3H. (C) The interaction of GST-ShHTL7 and HIS6-AtMAX2 were tested in the presence or absence of AtSMAX1-D2 in the AlphaScreen assay in response to 0.1 and 1 μM rac-GR24, with DMSO as the negative control. Error bars indicate se (n = 3). Two-way ANOVA analysis revealed significant differences between rac-GR24 concentrations and between presence and absence of AtSMAX1-D2 (P < 0.001). (D) AlphaScreen assay of the effect of AtSMAX1-D2 on the binding of GST-ShHTL7 to His6-AtMAX2 in response to GR24. A gradient concentration ranging from 3 to 3 × 10−5μM AtSMAX1-D2 was applied in the presence of 0.1 µM rac-GR24. The assay of GST-ShHTL7 and His6-AtMAX2 incubated with AtSMAX1-D2 in the presence of DMSO as the negative control. Error bars indicate se (n = 3). EC50 value was determined using nonlinear curve-fitting of graphs generated with Prism6 (GraphPad).