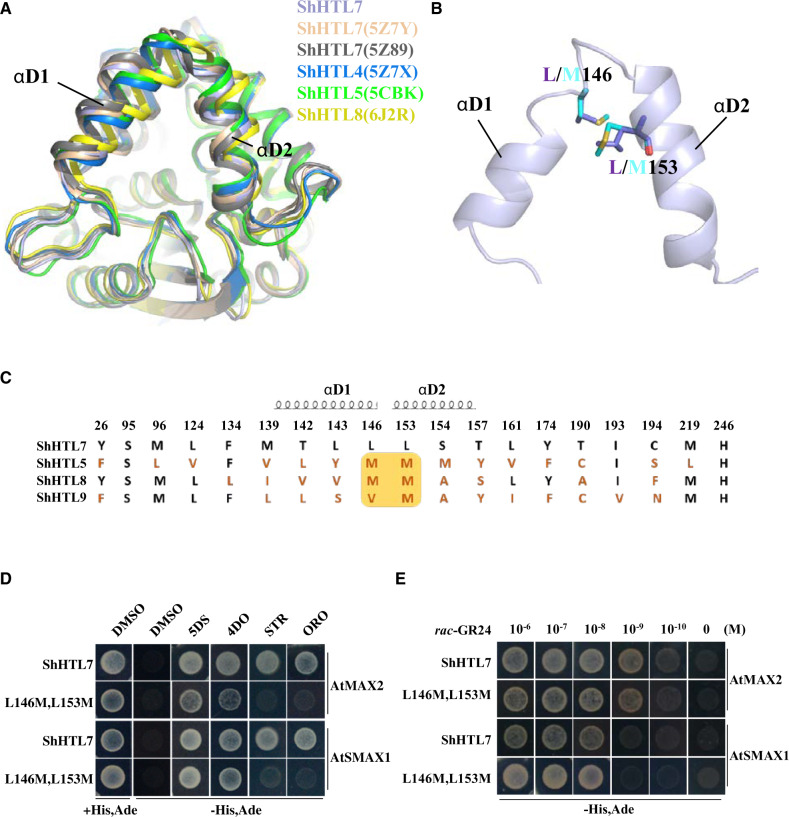
Figure 4.
ShHTL7 residues within helices αD1 and αD2 at the pocket entrance influence SL selectivity but not SL sensitivity in binding to AtMAX2 and AtSMAX1. (A) Alignment of ShHTL7 structures from this work (blue), previously determined ShHTL7 structures 5Z89 (gray) (Shahul Hameed et al., 2018) and 5Z7Y (pink) (Xu et al., 2018), ShHTL4 (5Z7X in dark blue), ShHTL5 (5CBK in green) (Xu et al., 2018), and ShHTL8 (6J2R in yellow) (Zhang et al., 2020). There are different shifts in the αD1 and αD2 helixes among the structures. (B) Sequence alignment of pocket amino acid residues for ShHTL7, ShHTL5, ShHTL8, and ShHTL9. Residues in red differ from those in ShHTL7. The yellow box highlights positions 146 and 153 that are most commonly M in other ShHTL proteins, in contrast to ShHTL7. Residues contained within helices αD1 and αD2 are indicated. (C) Location of residues L146 and L153 on αD1 and αD2 helices near the pocket entrance, and the structural changes associated with the L146M L153M double mutation. The ShHTL7 residues (L146 and L153) are shown in purple and mutant forms (M residues) in cyan. (D) Binding of BD-ShHTL7 and its double mutant (BD-L146M, L153M) to AD-AtMAX2 and AD-AtSMAX1 in response to 1 μM 5DS, 4DO, STR, and ORO in Y2H assays. (E) Interactions between BD-ShHTL7 or its double mutant BD-L146M, L153M with AD-AtSMAX1 or AD-AtMAX2 in response to rac-GR24 in the concentration range 10−6 to 10−10 M in Y2H assays.