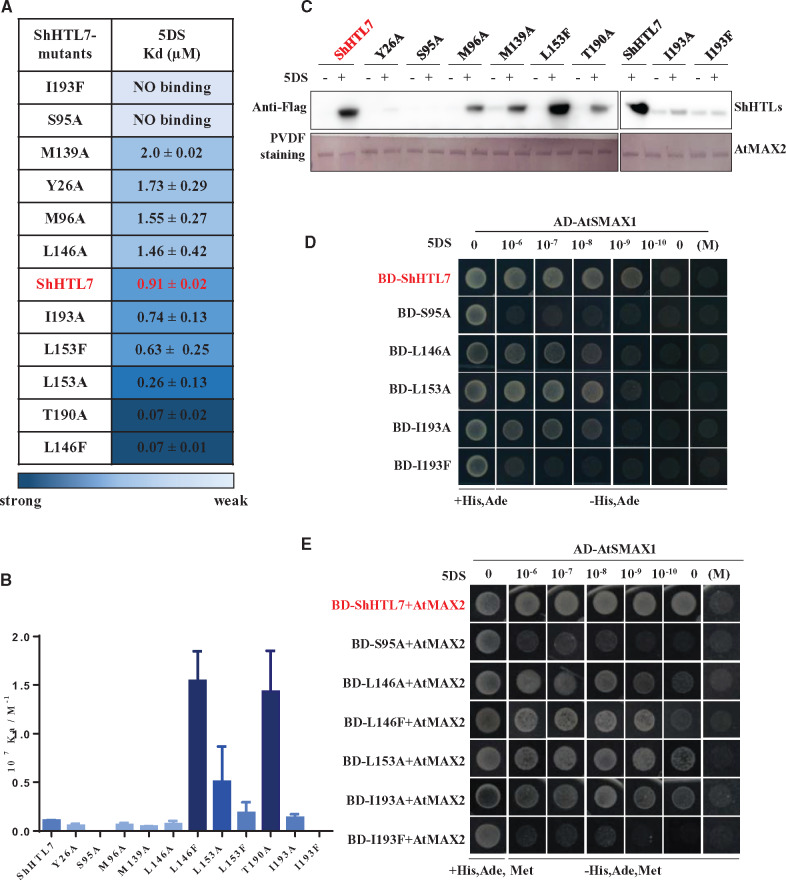
Figure 5.
ShHTL7 ligand binding affinity is not consistent with binding to AtMAX2 and AtSMAX1 or sensitivity to SL. A, Dissociation constants (Kd) for the binding of 5DS to ShHTL7 with mutations of pocket residues were measured by ITC assays. Kd values are means sd (n = >2). (B) Association constants (Ka) derived from (A). Values are means ± sd (n = >2). (C) In vitro pull-down assays of the interactions of His6-Flag-tagged ShHTL7 and its mutant forms with GST-AtMAX2 in response to 10 μM 5DS. The GST-AtMAX2 as bait was visualized by staining the PVDF membrane with Memstain to show loadings and the ShHTL7 proteins were detected with anti-Flag antibody. (D) Y2H assays of interactions between AD-AtSMAX1 and BD-ShHTL7 and its mutant forms in response to 5DS in the concentration range 10−6 to 10−10 M. (E) Y3H assays to determine the effect of AtMAX2 on the interactions shown in (D).