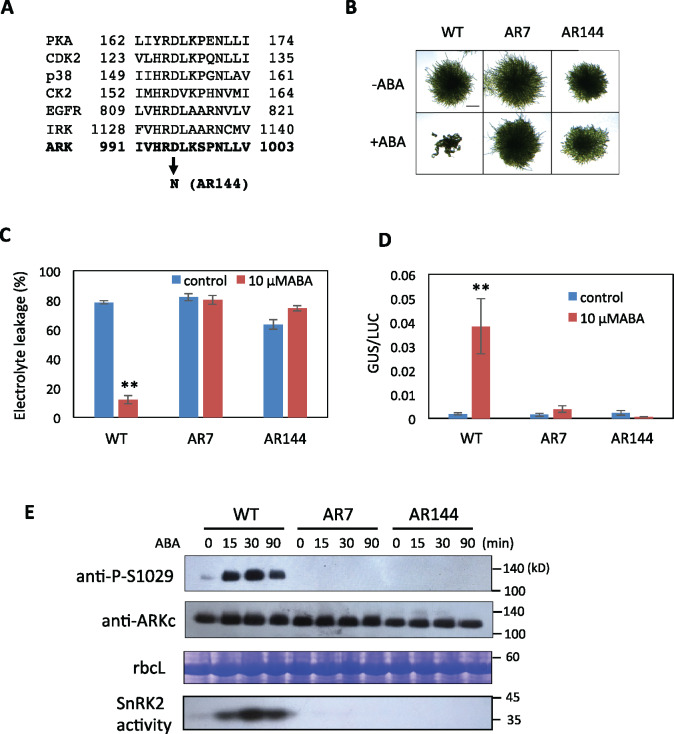
Figure 3.
ABA response of AR144 of P. patens with a mutation in the catalytic core of the kinase domain of ARK. (A) Comparison of amino acid sequences in the catalytic core of protein kinase A (PKA), cyclin-dependent protein kinase2 (CDK2), p38, casein kinase2 (CK2), and epidermal growth factor receptor (EGFR) (adapted from Nolen et al., 2004) with ARK. The conserved Asp (D) has been changed to Asn (N) in AR144. (B) Growth responses to ABA. Protonemata of WT, AR7, and AR144 were grown on a medium with 10-µM ABA for 1 week. (C) Comparison of freezing tolerance. Protonemata incubated with or without 10-µM ABA for 1 d were frozen to −4°C. After thawing, electrolyte leakage was measured to estimate the extent of freezing injury. Error bars indicate standard error of the mean. (D) Transient gene expression assays of WT, AR7, and AR144. Protonemata were bombarded with plasmid constructs of proEm-GUS and proUbi-LUC and were cultured with or without 10-µM ABA for 1 d for GUS and LUC assays. Error bars indicate standard error of the mean. **P < 0.01 in the t test (n = 3) compared with control WT without ABA treatment for (C) and (D). (E) Effect of ABA on Ser1029 phosphorylation in WT, AR7, and AR144. Protonemata were treated with 10-µM ABA for different times and used for immunoblot analysis with anti-phospho-Ser1029 (P-S1029) and anti-ARKc antibodies. Staining of the large subunit of ribulose bisphosphate carboxylase (rbcL) is shown as a control. Results of an in-gel kinase assay for detection of SnRK2 are also shown.