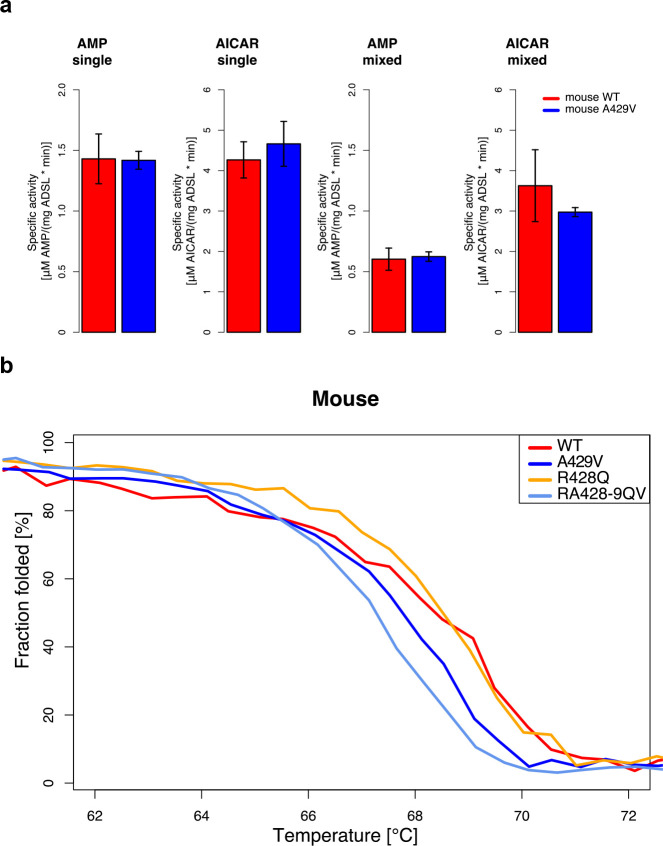
Figure 6. Characterization of mouse ADSL forms.
(a) Enzyme kinetics tested in substrate excess for the products (AICAR and AMP, see text above the plots) with one substrate (single) or with both substrates (mixed) in the reaction mix. Specific activities of wild type and humanized A429V versions do not differ (t-test, p>0.05). Error bars represent standard error of the mean from three experiments using two batches of proteins. (b) Protein melting measured by CD at 222 nm for WT, A429V, R428Q, and RA428-9QV ADSL versions. Lines represent the averages over four experiments. The A429V (t-test, p=0.10) and RA428-9QV versions are less stable than the other proteins (t-test, p=0.03, at denaturation midpoint (dm). dm(WT)=68.3 +/- 0.2°C, dm(A429V)=67.9 +/- 0.2°C, dm(R428Q)=68.6 +/- 0.0°C, and dm(RA428-9QV)=67.3 +/- 0.3°C).