Abstract
BACKGROUND AND PURPOSE: Although the prevalence of pineal cysts in autopsy series has been reported as being between 25% and 40%, MR studies have documented their frequency to range between 1.5% and 10.8%. The purpose of this high-resolution brain MR imaging study at 1.9T was to determine the prevalence of pineal cysts in healthy adults.
MATERIALS AND METHODS: Brain MR images of 100 healthy young volunteers were randomly selected from our International Consortium for Brain Mapping project data base. Cysts were detected as circular areas of isointensity relative to CSF on both 3D gradient-echo T1-weighted and 2D fast spin-echo T2-weighted images. The inner diameters of all visualized pineal cysts were measured, and a criterion of 2.0 mm of the largest inner cross-sectional diameter was used to categorize cysts as being either small cystic changes (<2.0-mm diameter) or pineal cysts (>2.0-mm diameter).
RESULTS: Twenty-three percent (23/100) of the volunteers had pineal cysts with a mean largest inner cross-sectional diameter of 4.3 mm (range, 2–14 mm); 13% (13/100) demonstrated cystic changes involving the pineal gland with the largest inner cross-sectional diameter of less than 2.0 mm. There was a slight female predominance. Two subjects with long-term follow-up scans showed no symptoms or changes in the size of their pineal cysts.
CONCLUSION: On high-resolution MR imaging, the prevalence of pineal cysts was 23% in our healthy group of adults, which is consistent with previous autopsy studies. Long-term follow-up studies of 2 cases demonstrated the stability of the cysts.
Presenting predominantly in adults in their 40s, benign cysts in the pineal gland are reported in people of all ages in 25% to 40% of autopsy series.1–3 These non-neoplastic glial cysts are uniloculated or multiloculated with a smooth wall.4 There can be a thin layer or nodule on the cyst wall from the pineal parenchymal tissue. Microscopic examination reveals that the cyst is composed of an inner layer of gliotic tissue, an intermediate layer of pineal parenchymal tissue, and an outer layer of connective tissue.4
A female predominance for large pineal cysts has been reported in some MR imaging studies.5–6 Most cysts are asymptomatic, with diameters ranging from 2 to 15 mm.5–8 When present, however, symptoms are usually noted in patients with cysts larger than 15 mm in diameter.9–10
The pineal cyst can occasionally enlarge the pineal gland and compress adjacent structures such as the superior colliculi of the quadrigeminal plate and the aqueduct of the midbrain, producing hydrocephalus and clinical symptoms.2 When present, the clinical symptoms include headache, vertigo, diplopia, blurred vision, hemiparesis, epilepsy, vomiting, bradycardia, papilledema, oculomotor nerve paresis, and Parinaud syndrome.2,11 Cases of pineal apoplexy that cause sudden death from an intracystic hemorrhage also have been reported.12–13
Several retrospective studies of consecutive MR imaging scans showed that the prevalence of cysts ranges from 1.5% to 10.8%.5–6,8,14–16 Most studies were conducted in patients with neurologic symptoms suggestive of an intracranial pathologic condition. One study, conducted among healthy volunteers aged between 22 and 40 years (13 men and 14 women) and using the conventional spin-echo T1-weighted sequence, reported the prevalence of benign pineal cysts to be 7.4%.17
The differential diagnosis of simple pineal cysts and cystic tumors, such as astrocytoma, pineocytoma, and pineoblastoma, is crucial for clinical management.7,18 Although there are no reliable diagnostic criteria for the differential diagnosis of such entities, most authors describe the benign pineal cyst as being well circumscribed and usually elliptical, with a slight hyperintensity or a similar signal intensity relative to the CSF on both the T1-weighted image (T1WI) and the T2-weighted image (T2WI).6,19–21 Enhancement of the cyst wall after the administration of a contrast agent is usually incomplete and not useful for the delineation of the extent of the capsule or the determination of the biologic nature of the lesion.22 On delayed contrast-enhanced MR imaging, pineal cysts may be enhanced uniformly as a solid mass.8,23 The mechanism of the uniform enhancement of the pineal cyst is not completely understood. The authors attribute this to either the passive diffusion of the MR imaging contrast agent from the wall of the pineal cyst or the active secretion of the contrast agent by the wall of the cyst.
The management of pineal cysts is still controversial.11 Mandera et al11 reported 4 cases of simple pineal cysts, all of which were treated surgically. One patient was symptom-free after surgery; preoperative symptoms in 2 patients cleared, except for light headaches. However, no improvement was seen in 1 of the 4 cases.
The natural history of the pineal cyst is not completely understood because no studies have been performed to follow the cyst from its appearance to its complete resolution. Nevertheless, some studies demonstrated that pineal cysts are stable during relatively long follow-up periods. Barboriak et al24 conducted a retrospective follow-up study in 32 patients with pineal cysts and other intracranial pathologic lesions. They observed that 75% of cysts remained stable over time, ranging from 0.5 to 9.1 years; 16% decreased in size or regressed completely, whereas 8% increased in size by 2.0–3.0 mm. Tamaki et al20 studied 31 subjects with pineal cysts; in 29 cases, the cysts did not change in size during the follow-up time from 3 months to 4 years. In 2 cases, the cysts spontaneously ruptured and collapsed during follow-up. In another study, Golzarian et al6 followed 12 subjects with pineal cysts; the cysts did not change in size throughout the 1-year follow-up. Finally, Bodensteiner et al17 reported 2 cases of pineal cysts in 27 healthy volunteers; the cysts did not change in size throughout the follow-up period of 6 months.
We performed this retrospective study, using high-resolution MR imaging at 1.9T, to determine the prevalence of pineal cysts in healthy adults.
Materials and Methods
High-resolution, noncontrast brain MR images (1.9T MRI scanner; Elscint, Haifa, Israel) of 100 healthy volunteers were randomly selected from our International Consortium for Brain Mapping (ICBM) project data base and retrospectively evaluated. The study population consisted of 70 men and 30 women, all aged between 19 and 39 years. The ICBM data base was prospectively collected at the Research Imaging Center at the University of Texas Health Science Center at San Antonio. The primary goal of the ICBM project was to develop a reference system on both macroscopic and microscopic levels on the structure and function of the human brain for the neuroscience community. The MR structural imaging as used in the current study was for building a normal reference anatomy of human brain.25
Institutional Review Board approval was obtained before the initiation of this study. After the volunteers signed the appropriate consent forms, we performed scans with the use of a 1.9 T MR imaging magnet (Elscint).
To determine eligibility for the ICBM study, we collected the subjects' health information, including a list of their past surgeries, allergies, and prescription and over-the-counter medicines, as well as the menstrual history of the female subjects. In addition, a neurologist performed complete physical, neurologic, and psychologic tests. A health screening form, filled out by each potential subject, and the results from the aforementioned health screening form and tests were used to select the subjects for this study. Only those without any history, sign, or symptom of neurologic or psychologic diseases were chosen to participate in the study.
We performed T1-weighted gradient echo 3D imaging (TR/TE = 24 ms/6 ms; tip angle = 25°; FOV = 25.6 × 25.6 cm; matrix size = 256 × 256; section thickness = 1 mm; number of average = 1) and T2-weighted fast spin-echo imaging (TR/TE = 4200 ms/128 ms; echo-train length = 14; FOV = 25.6 × 25.6 cm; matrix size = 256 × 256; section thickness = 2 mm; number of average = 1; gap = 0). An MR imaging contrast agent was not administered in any of the cases. Two neuroradiologists interpreted all of the MR images of this study electronically on the MR imaging console monitor. Measurements of the pineal cysts also were done electronically on the MR imaging console with the scanner vendor-provided standard MR imaging software. The internal structure, MR imaging signal intensity, and margins of the cysts were evaluated as well as the mass effect of the cyst on the adjacent midbrain structures, including the aqueduct of the midbrain and the superior colliculi of the quadrigeminal plate. Categorizing the cysts according to their cross-sectional inner diameter and setting a 2.0-mm criterion, we divided the cysts into small cystic changes (<2.0-mm diameter) and pineal cysts (>2.0-mm diameter). Two millimeters was used as a cutoff criterion for the pineal cysts because the smallest reported pineal cyst in MR imaging studies was 2.0 mm.8 Finally, follow-up MR imaging scans were performed on 2 subjects, one 8 years and 7 months and the other 9 years and 9 months after the initial scans.
Results
On the MR imaging, the pineal cysts were sharply delineated, ovoid-shaped lesions in the pineal gland, without intracystic trabeculations. Their MR imaging signal intensities on both the T1WI and the T2WI were similar to those of the CSF in the ventricles. We observed the prevalence rate of the cysts to be 23% (9 women and 14 men in 100 healthy volunteers), with a slight female predominance (9/30 women vs 14/70 men). However, the female predominance was not statistically significant (P = .054). The mean of the largest inner cross-sectional diameter of the cysts was 4.3 mm, with a range of 2–14 mm. Thirteen percent of the subjects (8 men and 5 women) had small cystic changes (less than 2.0 mm in diameter) in the pineal glad that also were isointense on the T1WI and T2WI relative to the CSF. There was a slight female predominance in the cystic changes (5/30 women vs 8/70 men), again without statistical significance (P = .74). However, for the combined presence of cysts and cystic changes observed, a female predominance was statistically significant (14/30 women vs 22/70 men, P < .05). The typical pineal cyst and cystic changes are shown in Figs 1 and 2. In 2 of the 36 cases, the pineal cysts touched the superior colliculi of the quadrigeminal plate without causing any deformity. However, there was no evidence of an obstructive hydrocephalus or of a narrowing of the aqueduct. All subjects with and without pineal cysts were completely asymptomatic.
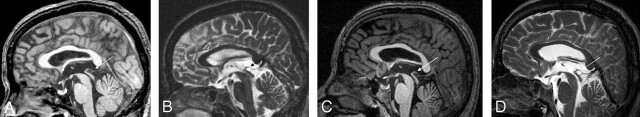
Figure 1.
Large pineal cyst in a healthy 38-year-old man. A, Sagittal noncontrast T1WI (gradient-echo; TR/TE/flip angle = 24 ms/6 ms/25°). B, Axial T2WI (fast spin-echo; TR/TE/echo-train length = 4200 ms/128 ms/14). C, Sagittal noncontrast follow-up T1WI, 8 years and 7 months after initial scans. D, Sagittal noncontrast follow-up T2WI, 8 years and 7 months after initial scans. The arrows indicate the pineal cyst. The pineal cyst touches the superior colliculi of the quadrigeminal plate without causing any deformity. However, no evidence of an obstructive hydrocephalus or of a narrowing of the aqueduct of the midbrain is seen.
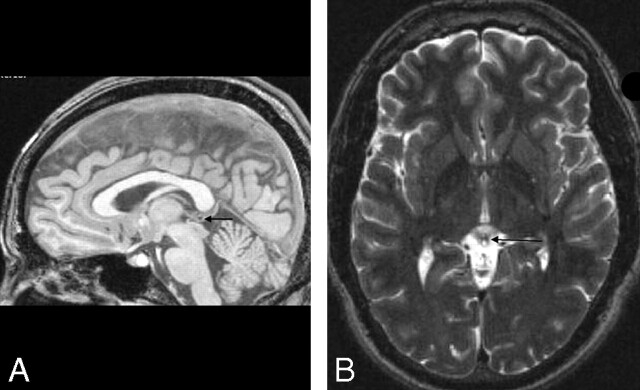
Figure 2.
Small cystic change in the pineal gland of a healthy 25-year-old man. A, Sagittal noncontrast T1WI (gradient echo; TR/TE/flip angle = 24 ms/6 ms/25°). B, Axial T2WI (fast spin-echo; TR/TE/echo-train length = 4200 ms/128 ms/14). The arrows point to the cystic change in the pineal gland.
Two subjects with large pineal cysts had follow-up scans, one 8 years and 7 months after and the other 9 years and 9 months after the initial scans. The largest inner cross-sectional diameters of their cysts were 8.6 mm for one and 12.9 mm for the other. They have been completely asymptomatic during the follow-up period, and their pineal cysts did not change in size, signal intensity, or internal structure on the follow-up images (Fig 1).
Discussion
Our study demonstrated a much higher pineal cyst prevalence of 23% in healthy persons compared with that observed in previous MR imaging studies conducted in either patients with various neurologic symptoms for medical indications5–6,14–16 or healthy volunteers.17 Thirteen percent of subjects in this study demonstrated cystic changes in the pineal gland. A possible explanation for the difference between the prevalence of the pineal cysts demonstrated in our study and that in other MR imaging studies is that we used high-resolution MR imaging with a 1.0 mm resolution on the 3D T1WI and an in-plane resolution of 1.0 mm with an axial resolution of 2.0 mm on the T2WI. An in-plane resolution of 1.0 mm and an axial resolution of 3.0 mm were used in the prior MR imaging study of healthy volunteers.17 Because the smallest reported pineal cyst was 2.0 mm,8 we classified MR imaging signal intensity changes compatible with pineal cysts but less than 2.0 mm in diameter as being pineal cystic changes; they were not considered pineal cysts. The high prevalence rate of pineal cysts and cystic changes demonstrated in this study is consistent with prior autopsy studies that showed macroscopic (measuring up to 0.5 × 0.3 × 0.2 cm) and microscopic cysts in 21.8% and 17.9% of patients, respectively, who died from various diseases in 168 consecutive cases.3
Physiologic calcifications in the pineal gland are very common and vary with age.26 Although they have both low and high MR imaging signal intensities on the T1WI,27–29 they should be hypointense relative to CSF on the T2WI because of the T2 relaxation shortening and the low proton attenuation in the calcifications. Because the MR imaging signal intensity characteristics of the cysts and the cystic changes demonstrated in this study were similar to those of CSF, pineal cysts, and cystic changes should not have been confused with pineal calcifications.
Our study demonstrated that there is a slight female predominance in the occurrence of pineal cysts and pineal cystic changes, which is consistent with the results of prior retrospective MR imaging studies conducted for other medial indications5–6; however, a female predominance was not shown in the prior study of healthy subjects.17 One possible explanation for this difference could be the relatively small sample size in the other study's healthy group, with only 2 cases of pineal cysts identified in 27 subjects. In addition, this difference could be related to the method of patient recruitment. Further study may be warranted to address this difference with a large healthy subject population.
The most important implication of the high prevalence of asymptomatic pineal cysts in healthy subjects demonstrated in this study is that no surgical intervention or imaging follow-up is warranted for such cysts because 23% of healthy subjects have such cysts, and primary intracranial tumors in the pineal region represent only 1.0% of all intracranial tumors.26 Small pineal cysts should be treated as normal variants, especially in female subjects, because of the slight female predominance in pineal cysts and pineal cystic changes.
Conclusion
The prevalence of asymptomatic pineal cysts in healthy adults on high-resolution MR imaging is 23%, which is consistent with previous autopsy studies. The stability of pineal cysts was demonstrated in 2 of the subjects on follow-up studies. This study suggests that further work-up, imaging follow-up, or therapeutic intervention is unwarranted for small and asymptomatic cysts in otherwise healthy adults.
Acknowledgments
We are grateful to Dr. Lin Xiong for proving follow-up MR images.
Footnotes
This research was supported by the Human Brain Mapping Project jointly funded by the National Institute of Mental Health and National Institute on Drug Abuse (9 P01-EB001955-12).
Previously presented in part at: Neuroradiology Education and Research Foundation Symposium 2006 and Annual Meeting of the American Society of Neuroradiology, April 29–May 5, 2006; San Diego, Calif.
References
- 1.Tapp E, Huxley M. The histological appearance of the human pineal gland from puberty to old age. J Pathol 1972;108:137–44 [DOI] [PubMed] [Google Scholar]
- 2.Tapp E. The histology and pathology of the human pineal gland. Prog Brain Res 1979;52:481–500 [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa A, Ohtsubo K, Mori W. Pineal gland in old age; quantitative and qualitative morphological study of 168 human autopsy cases. Brain Res 1987;409:343–49 [DOI] [PubMed] [Google Scholar]
- 4.Hirato J, Nakazato Y. Pathology of pineal region tumors. J Neurooncol 2001;54:239–49 [DOI] [PubMed] [Google Scholar]
- 5.Di Costanzo A, Tedeschi G, Di Salle F, et al. Pineal cysts: an incidental MRI finding? J Neurol Neurosurg Psychiatry 1993;56:207–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golzarian J, Baleriaux D, Bank WO, et al. Pineal cyst: normal or pathological? Neuroradiology 1993;35:251–53 [DOI] [PubMed] [Google Scholar]
- 7.Fetell MR, Bruce JN, Burke AM, et al. Non-neoplastic pineal cysts. Neurology 1991;41:1034–40 [DOI] [PubMed] [Google Scholar]
- 8.Jinkins JR, Xiong L, Reiter RJ. The midline pineal “eye”: MR and CT characteristic of the pineal gland with and without benign cyst formation. J Pineal Res 1995;19:64–71 [DOI] [PubMed] [Google Scholar]
- 9.Fain JS, Tomlinson FH, Scheithauer BW, et al. Symptomatic glial cysts of the pineal gland. J Neurosurg 1994;80:454–60 [DOI] [PubMed] [Google Scholar]
- 10.Klein P, Rubinstein LJ. Benign symptomatic glial cysts of the pineal gland: report of seven cases and review of the literature. J Neurol Neurosurg Psychiatry 1989;52:991–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandera M, Marcol W, Bierzynska-Macyszyn G, et al. Pineal cysts in childhood. Childs Nerv Syst 2003;19:750–55 [DOI] [PubMed] [Google Scholar]
- 12.Milroy CM, Smith CL. Sudden death due to a glial cyst of the pineal gland. J Clin Pathol 1996;49:267–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson JK, Hirsch CS. Sudden, unexpected death due to pineal apoplexy. Am J Forensic Med Pathol 1986;7:64–68 [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Norman D, Newton TH. MR imaging of pineal cysts. J Comput Assist Tomogr 1987;4:586–90 [DOI] [PubMed] [Google Scholar]
- 15.Lum GB, Williams JP, Machen BC, et al. Benign cystic pineal lesions by magnetic resonance imaging. J Comput Tomogr 1987;11:228–35 [DOI] [PubMed] [Google Scholar]
- 16.Mamourian AC, Towfighi J. Pineal cysts: MR imaging. AJNR Am J Neuroradiol 1986;7:1081–86 [PMC free article] [PubMed] [Google Scholar]
- 17.Bodensteiner JB, Schaefer GB, Keller GM, et al. Incidental pineal cysts in a prospectively ascertained normal cohort. Clinical Pediatrics 1996;35:277–79 [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama1 K, Arita K, Okamura1 T, et al. Detection of a pineoblastoma with large central cyst in a young child. Childs Nerv Syst 2002;18:157–60 [DOI] [PubMed] [Google Scholar]
- 19.Fleege A, Miller GM, Fletcher GP, et al. Benign glial cysts of the pineal gland: unusual imaging characteristic with histologic correlation. AJNR Am J Neuroradiol 1994;15:161–67 [PMC free article] [PubMed] [Google Scholar]
- 20.Tamaki N, Shirataki K, Lin T, et al. Cysts of the pineal gland. A new clinical entity to be distinguished from tumors of the pineal region. Childs Nerv Syst 1989;5:172–76 [DOI] [PubMed] [Google Scholar]
- 21.Welton PL, Reicheer MA, Kellerhouser LE, et al. MR of benign pineal cyst. AJNR Am J Neuroradiol 1988;9:612. [PMC free article] [PubMed] [Google Scholar]
- 22.Barkovich JA. Pediatric neuroimaging. In: Norman D, ed. Contemporary Neuroimaging. New York: Raven Press;1990. :193–95
- 23.Mamourian AC, Yarnell T. Enhancement of pineal cysts on MR images. AJNR Am J Neuroradiol 1991;12:773–74 [PMC free article] [PubMed] [Google Scholar]
- 24.Barboriak DP, Lee L, and Provenzale JM. Serial MRI imaging of pineal cysts: implications for natural history and follow-up. AJR Am J Roentgenol 2001;176:737–43 [DOI] [PubMed] [Google Scholar]
- 25.Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 2001;356:1293–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman RI, Yousem DM. Neuroradiology: The Requisites. Philadelphia: Mosby;2003. :156–62
- 27.Narita K, Murata T, Ito T, et al. A case of diffuse neurofibrillary tangles with calcification. Psychiatry Clin Neurosci 2002;56:117–20 [DOI] [PubMed] [Google Scholar]
- 28.Dell LA, Brown MS, Orrison WW, et al. Physiologic intracranial calcification with hyperintensity on MR imaging: case report and experimental model. AJNR Am J Neuroradiol 1988;9:1145–48 [PMC free article] [PubMed] [Google Scholar]
- 29.Henkelman RM, Watts JF, and Kucharczyk W. High signal intensity in MR images of calcified brain tissue. Radiology 1991;179:199–206 [DOI] [PubMed] [Google Scholar]