Abstract
BACKGROUND AND PURPOSE: Anterior cerebral artery (ACA) emboli may occur before or during fibrinolytic revascularization of middle cerebral artery (MCA) and internal carotid artery (ICA) T occlusions. We sought to determine the incidence and effect of baseline and new embolic ACA occlusions in the Interventional Management of Stroke (IMS) studies.
MATERIALS AND METHODS: Case report forms, pretreatment and posttreatment arteriograms, and CTs from 142 subjects entered into IMS I & II were reviewed to identify subjects with baseline ACA occlusion, new ACA emboli occurring during fibrinolysis, subsequent CT-demonstrated infarction in the ACA distribution, and to evaluate global and lower extremity motor clinical outcome.
RESULTS: During M1/M2 thrombolysis procedures, new ACA embolus occurred in 1 of 60 (1.7%) subjects. Baseline distal emboli were identified in 3 of 20 (15%) T occlusions before intra-arterial (IA) treatment, and new posttreatment distal ACA emboli were identified in 3 subjects. At 24 hours, 8 (32%) T occlusions demonstrated CT-ACA infarct, typically of small volume. Infarcts were less common following sonography microcatheter-assisted thrombolysis compared with standard microcatheter thrombolysis (P = .05). Lower extremity weakness was present in 9 of 10 subjects with ACA embolus/infarct at 24 hours. The modified Rankin 0 to 2 outcomes were achieved in 4 of 25 (16%) subjects with T occlusion overall, but in 0 of 10 subjects with distal ACA emboli or ACA CT infarcts (P = .07).
CONCLUSIONS: With IV/IA recombinant tissue plasminogen activator treatment for MCA emboli, new ACA emboli are uncommon events. Distal ACA emboli during T-occlusion thrombolysis are not uncommon, typically lead to small ACA-distribution infarcts, and may limit neurologic recovery.
Distal arterial emboli may be sequelae of intravenous (IV) and intra-arterial (IA) fibrinolysis of the middle cerebral artery (MCA) and distal internal carotid artery (ICA) “T” occlusions as the initial thrombus fragments.1 Distal emboli may result in reduction of collateral blood flow and new infarctions that affect neurologic outcome. The clinical significance of secondary distal emboli liberated during therapeutic recanalization of MCA and ICA T occlusions has not been analyzed. It is intuitive that different paradigms of revascularization may have different effects on the creation and clinical effect of such emboli in the anterior cerebral artery (ACA). To better understand these effects, we examined the prevalence of ACA territory emboli (primary and secondary) and infarctions and correlated these with clinical outcomes in subjects with MCA and ICA T occlusions treated with combined IV and IA fibrinolysis in the Interventional Management of Stroke (IMS) I and II studies.
Materials and Methods
A cohort of combined IV and IA cases were reviewed from the IMS I and II studies. The IMS I trial was an 80-subject, 17-center, open-labeled, single-arm pilot study to investigate the feasibility and safety of combined IV and IA recombinant tissue plasminogen activator (rtPA) treatment of ischemic strokes.2 IMS II was a 73-subject continuation of IMS I, with the additional intention of treating appropriate occlusions with an IA microcatheter infusing the rtPA while delivering a low-intensity sonography within the thrombus to accelerate thrombolysis.3,4
In brief, subjects with acute ischemic stroke defined by a baseline National Institutes of Health Stroke Scale (NIHSS) score ≥10 were enrolled within 3 hours of the onset of symptoms and treated with IV tPA at a reduced dose (0.6 mg/kg over 30 minutes with 15% of the dose given as an initial bolus). They were then taken immediately to the neuroangiography suite for endovascular therapy. If a thrombus was identified within a cerebral artery, IA treatment with up to 22 mg of IA rtPA was administered through a microcatheter placed in the thrombus. Control cerebral angiograms were performed every 15 minutes. In IMS I, manipulation of the thrombus by advancement or retraction of the microcatheter or microguidewire was allowed at each 15-minute control angiogram. In IMS II, the sonography microcatheter was to be placed into the proximal aspect of the thrombus, with no manipulation of the guidewire, but incrementally advanced as thrombolysis was demonstrated on subsequent control arteriograms.
Determination of ACA Territory Occlusions and Infarctions
Case report form data, pretreatment and posttreatment arteriograms, and 24-hour CT scans for 142 IMS I and II subjects with anterior circulation ischemia were reviewed to identify emboli and infarctions in the ACA territory. In subjects with M1 or M2 occlusion, preexisting ACA emboli and postfibrinolysis ACA emboli were recorded. In subjects with ICA T occlusion and absent filling of the ipsilateral A1 segment, contrast injection into the opposite carotid artery was examined for cross-filling through the anterior communicating artery and emboli in the A2 segment or distal ACA. Preexisting ACA emboli and postfibrinolysis ACA emboli were recorded, as well as time-to-IA treatment. Case report forms were also reviewed to determine 3-month clinical outcome on the basis of the modified Rankin Scores (mRS), as well as contralateral lower extremity weakness (item 6 of the NIHS). Complete rates of recanalization (grade 3) of the primary arterial occlusive lesion (AOL) were recorded, and perfusion of the distal MCA was graded by a modified thrombolysis in cerebral infarction (TICI) score.5,6 TICI perfusion was graded as no perfusion (grade 0); perfusion past initial obstruction, but limited distal branch flow (grade 1); perfusion of one half or more of the original distribution of the occluded artery (grade 2); and normal perfusion or flow through all segments (grade 3).
Statistical Analysis
Secondary posttreatment embolic occlusions and CT-demonstrated cerebral infarctions in the territory of the ACA were correlated with clinical outcome and treatment paradigm (standard microcatheter treatment in IMS I and II versus the use of a sonography microcatheter [EKOS, Bothell, Wash] in IMS II). Clinical outcome was assessed according to the mRS, with a good clinical outcome being a mRS of 0 to 2. In the ICA T-occlusion group, subgroup analysis was also performed on the basis of the presence or absence of hemodynamically significant occlusive disease of the ipsilateral extracranial ICA (occlusion or >70% stenosis). The significance of each association was tested with χ2 analysis, where P = <.05 is considered a significant relationship.
Results
Prevalence of ACA Occlusions before IA Treatment
ACA occlusions were identified on baseline arteriograms in 32 of 142 (22.5%) subjects. Isolated ACA emboli were identified in 2 (1.4%). ACA emboli in combination with MCA emboli occurred in 5 (3.5%) subjects.
ICA T occlusion with ipsilateral A1 occlusion was identified in 25 (17.9%) subjects. Of 25 ICA T occlusions, 21 (84%) had imaging of the opposite carotid artery allowing evaluation of the distal (A2 or beyond) ACA on the side of the ICA T occlusion. A total of 20 of 21 (95.2%) subjects exhibited cross-filling. Where cross-filling was present, distal ACA emboli in the A2 segment or beyond were identified in 3 of 21 (14%) T occlusions before IA treatment. Eight subjects with T occlusions had concurrent cervical ICA stenosis >70% (n = 7) or occlusion (n = 1). No angioplasty or stent placement was performed in these 8 subjects.
CT Infarcts and Clinical Outcome of ACA Occlusions after IA Treatment
Of 2 isolated A2 emboli on baseline arteriograms, 1 was treated intra-arterially, and 1 was not. Both exhibited small 24-hour CT infarct, but both achieved 0 to 2 outcomes on mRS. Of 5 A2–3 occlusions with MCA occlusion treatment, no ACA local treatment was administered. MCA occlusion of 3 of 5 subjects was treated with IA rtPA, and all 3 subjects exhibited small CT-ACA infarcts at 24 hours, achieving mRS of 2, 4, and 6 at 3 months.
During M1/M2 thrombolysis procedures, a new ACA embolus occurred in 1 of 60 (1.7%) subjects, with ACA infarction on CT at 24 hours (Fig 1).
Fig 1.
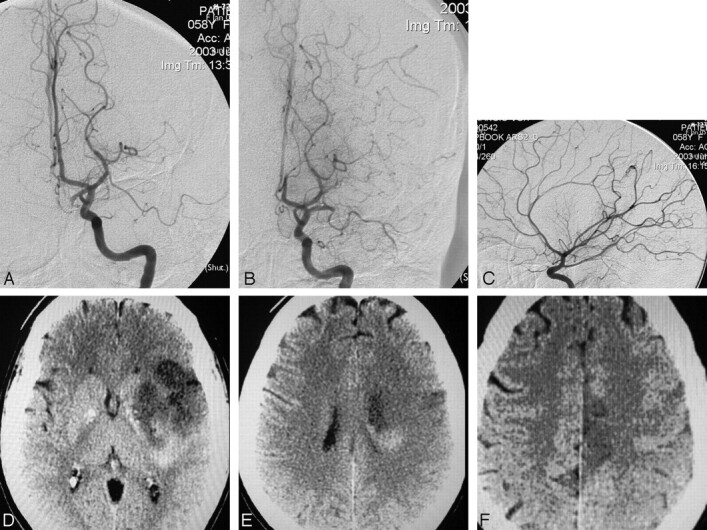
A, Pretreatment arteriogram with M1 occlusion and patent ACA. Baseline NIHSS 25. B, Control arteriogram after 45 minutes of IA treatment demonstrates A2 occlusion, with partial M1 recanalization. C, Final arteriogram after IA treatment demonstrates persistent occlusion of the distal left pericallosal artery superimposed on partial filling of the right ACA, complete recanalization of the M1 trunk with TICI 2 perfusion. D–F, A 24-hour CT demonstrates deep, insular, and inferior frontal infarct. MCA infarct extends superiorly (E) toward the distal posterior frontal lateral cortical borderzone and the distal ACA infarct (F).
During ICA T-occlusion thrombolysis, complete recanalization of the ICA segment (allowing evaluation of distal antegrade flow for distal embolization) occurred in 15 (60%) subjects, with normal A1 segment flow in 11 (44%), and partial A1 patency with flow in 3 (12%), in addition to 36% TICI II distal MCA perfusion (Table). New posttreatment distal ACA emboli (A2 or more distal) were subsequently identified in 3 of 20 (15%) subjects, all of whom had complete recanalization of the ICA and A1 segment. There were 2 of 3 new emboli in the ICA stenosis or occlusion group. Time to IA treatment was similar in the ACA emboli group (both pretreatment and posttreatment, n = 6) compared with the non-ACA embolus (n = 19) group.
Analysis of recanalization and clinical outcomes of ICA T occlusions in IMS 1 and II
| Thrombolytic | N | Cross-filling | ACA Embolus Pretreatment | Complete Recanalization (Grade 3) | TICI Perfusion (Grades 2,3) | New ACA Embolus Posttreatment | CT Infarct | R 0–2 |
|---|---|---|---|---|---|---|---|---|
| IV + IA rtPA | 25 | 20/21 (95.2%) | 3/20 (15%) | 15/25 (60%) | 9/25 (36%) | 3/20 (15%) | 8/25 (32%) | 4/25 (16%) |
Note:—ACA indicates anterior cerebral artery; R 0–2, modified Rankin scores; IV + IA, intravenous and intra-arterial; rtPA, recombinant tissue plasminogen activator; TICI, Thrombolysis in Cerebral Infarction score.
Four subjects with grade 0 or grade 1 recanalization (no perfusion) did not have the opposite ICA injected after the procedure, so the A2 or distal emboli could not be identified. One subject exhibited a CT-ACA infarct (Fig 2D).
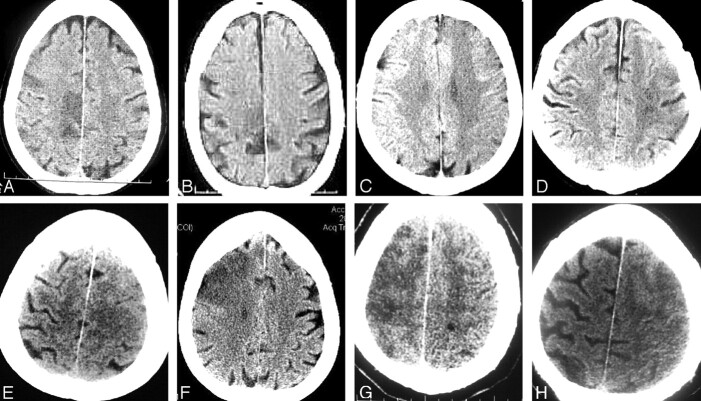
Fig 2.
Single 24-hour CT images from each of 8 subjects demonstrates ICA T occlusion with infarcts in the ACA distribution. Infarcts were all of small volume, located in the ACA cortical areas (A, B), cortical with subjacent white matter and internal borderzone areas (C–E), or in continuity with larger MCA infarct demonstration (F–H).
At 24 hours, 8 (32%) ICA T CT-ACA occlusions demonstrated CT-ACA infarct, typically of small volume (Fig 2). None were judged due to cingulate herniation. Infarcts were pure cortical, subjacent white matter and internal borderzone, with or without direct continuity with MCA distribution infarcts. Infarcts were demonstrated in 2 of 3 subjects with baseline distal emboli, and 2 of 3 subjects with new emboli. Of the remaining 4 infarcts, 3 occurred without angiographic identification of emboli, and 1 occurred in a subject with no baseline cross-filling and no recanalization. CT-demonstrated ACA infarcts were less common after MicroLysUS-assisted thrombolysis (EKOS) (n = 9) in IMS II, compared with standard microcatheter thrombolysis in IMS I and IMS II (P = .05).
Good 3-month clinical outcomes (mRS 0–2) were achieved in 4 of 25 (16%) T-occlusion subjects, but in none of the 10 subjects with distal ACA emboli or CT-ACA infarcts (P = .07). Of 10 subjects with ACA emboli or CT-ACA infarct, 9 exhibited contralateral lower extremity weakness on Item 6 of the NIHSS at 24 hours. Of 5 surviving subjects, 4 exhibited contralateral leg weakness at 3 months.
Of the 4 subjects without injection of the opposite ICA allowing demonstration of the A2 segment on the involved side, one Rankin 2 outcome was achieved, and that was in the absence of an ACA-distribution CT infarct.
Discussion
New emboli introduced into the ACA have been infrequently reported with IV or IA fibrinolysis. With M1 or M2 occlusion, fragmentation with new emboli into the distal MCA may occur as a by-product of revascularization of the proximal occlusion. Our review of ACA embolization in IMS I and II confirms that ACA emboli after MCA thrombolysis are indeed uncommon, with only 1 occurrence in 60 (1.7%) subjects with M1 or M2 occlusions treated with combined IV and IA therapy. No new ACA emboli were reported in 120 treated subjects in the PROACT II study, in which no catheter or microguidewire thrombus manipulation of the MCA occlusion was allowed.7 The MERCI study, which examined revascularization with a new mechanical device with or without an adjuvant IA thrombolytic, reported 3 new ACA emboli in 80 (3.8%) subjects with MCA occlusion.8 Similar embolic complications have been reported after balloon angioplasty,9–13 and additional reports have even suggested that IV tPA may be a safe and efficacious treatment technique for the small distal fragments involving the MCA divisions.14,15 The relatively low occurrence of ACA emboli after combined IV and IA treatment of MCA occlusions in the IMS paradigm, compared with the higher prevalence seen with these more aggressive methods of revascularization, suggests that mechanical manipulation may play a part in the production of ACA emboli.
Identification of additional new emboli during ICA T-occlusion therapy further suggests that mechanical manipulation increases production of ACA emboli. CT-demonstrated infarcts were less common after MicroLysUS-assisted thrombolysis (n = 9) compared with standard microcatheter thrombolysis (P = .05) of ICA T occlusions. MicroLysUS-assisted thrombolysis called for placing the microcatheter into the presenting face of the thrombus, without continuing distal to the thrombus, and without microguidewire manipulation of the thrombus. Standard microcatheter-based fibrinolysis protocol allowed manipulation of the thrombus at 15-inch intervals with advancement or retraction of the microcatheter and manipulation of the microguidewire. Although this reduced identification of an ACA embolus may be a function of some intrinsic advantage of sonography-assisted thrombolysis, such as quicker or more complete thrombolysis, it may also reflect a disadvantage of more aggressive thrombus manipulation in creating ACA emboli.
No previous report of ICA T occlusion has detailed the prevalence of pre-existing ACA emboli, or new distal emboli during treatment. Successful mechanical thrombus manipulation of distal ICA occlusions has been emphasized, recognizing the potential for distal embolizations, but their incidence was not recorded.1,16,17 Arnold18 reported IA treatment of 24 ICA T occlusions but only addressed recanalization of the A1 segment, without providing details regarding distal ACA emboli. Zaidat19 compared IV and IA to IA thrombolytic treatment for distal ICA occlusions in 18 patients but did not focus on distal ACA occlusive changes or CT-ACA infarcts.
Prognostic factors affecting long-term outcome after thrombolytic therapy include age, severity of the stroke, location of emboli, time to treatment, and rates of recanalization.20–26 Recent focused analysis has shown that leptomeningeal collateralization correlates strongly with long-term outcome in acute ischemic stroke.20,27 ACA emboli with ICA T occlusion can affect outcome not only by causing new infarcts superimposed on the typically large deep and MCA distribution infarct (Fig 2A–E), but also by reducing collateral flow, shifting or enlarging the infarct borderzone (Fig 2F–H). Besides supplying ischemic tissue with blood, Caplan28 suggested that collateral blood flow may allow greater exposure to the thrombolytic drug and clearance of thrombus fragments during fibrinolysis, both of which may be presumably compromised in the event of showering distal emboli to the ACA. Furthermore, besides providing blood to external borderzone territories, Bisschops29 reported that unilateral ICA occlusions with collateral blood flow through the anterior communicating artery demonstrated a significant reduction in volume and prevalence of internal borderzone infarctions compared with the absence of collateral flow through the anterior communicating artery. We believe that the demonstration of cross-filling in 20 of 21 of our subjects with T occlusions, with subsequent small ACA infarcts, may be a confirmatory reflection of the importance of collateral circulation.
This analysis also suggests clinically significant effects of ACA emboli with treatment of ICA T occlusions, despite the small sizes of the infarcts at 24 hours. Nine of 10 subjects with ACA emboli or infarct exhibited leg weakness at 24 hours, as did 4 of 5 surviving to 3 months. No good outcomes were attained in the subjects with ACA emboli or infarcts. Despite the small size of some ACA infarcts, they can be quite devastating to functional status, particularly when combined with MCA infarcts. Paralysis of the contralateral leg and associated immobility can severely impair recovery and contributes to complications of stroke including pneumonia and pulmonary thromboembolism. Neuropsychologic dysfunction including dysexecutive syndromes, akinetic mutism, altered control of the sphincter, and amotivational syndromes can lead to severe disability even in the presence of a motor recovery.
The high incidence of lower extremity neurologic deficit and the absence of good outcomes in the presence of an ACA embolus or infarct make it reasonable to suggest that ACA emboli should be avoided whenever possible, unless some other clear clinical benefit can be shown to accrue in concert with their production during a revascularization procedure, or with a particular revascularization paradigm. Although distal emboli have correlated with worse clinical outcome elsewhere,27,30,31 ACA emboli may be less significant than failure of timely restoration of blood flow in influencing the Rankin outcome score. The demonstration of absence, hypoplasia, or occlusion of the anterior communicating artery on pretreatment angiograms, with the absence of collateral flow from the contralateral ACA under these conditions, may decrease the negative impact of production of distal embolizations to the ACA territory, but only 1 such instance was identified in our population.
The small size of ACA infarcts at 24 hours is notable, despite the absence of cross-filling in 1 subject. We hypothesize that the combined therapy paradigm, with early administration of IV rtPA maintaining or creating collateral flow, while lysing some small distal emboli, may, in fact, be responsible for limiting the volume of the infarct. Comparison of an untreated control group of patients with proved ICA T occlusion, as well as with those treated by other methods (an analysis in progress), would be required to substantiate that hypothesis further.
Conclusions
With IV and IA rtPA treatment for MCA emboli in IMS I and IMS II, new ACA emboli were uncommon events. Distal ACA emboli during fibrinolysis of an ICA T occlusion are more common, typically leading to small ACA-distribution infarcts. Distal ACA infarcts may be less frequent with sonography-assisted thrombolysis. ICA-T occlusion with distal ACA emboli seems to adversely affect neurologic recovery. Observations and results reported here may pertain to combined IV and IA therapy only, but attention to these occurrences in future trial analysis may allow comparisons with other treatment paradigms.
Acknowledgments
IMS I and II were funded by the National Institute of Neurologic Diseases and Stroke (NINDS #NS39160). The rt-PA was supplied by Genentech. In IMS I, microcatheters were supplied by Cordis Neurovascular. In IMS II, EKOS Micro-Infusion Systems were supplied by EKOS Corporation.
References
- 1.Qureshi AI, Siddiqui AM, Suri MF, et al. Aggressive mechanical clot disruption and low-dose intra-arterial third-generation thrombolytic agent for ischemic stroke: a prospective study. Neurosurgery 2002;51:1319–27; discussion 1327–29 [DOI] [PubMed] [Google Scholar]
- 2.IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–11 [DOI] [PubMed] [Google Scholar]
- 3.IMS II trial investigators. The International Management of Stroke (IMS) II study. Stroke 2007;38:2127–35 [DOI] [PubMed] [Google Scholar]
- 4.Mahon BR, Nesbit GM, Barnwell SL, et al. North American clinical experience with the EKOS MicroLysUS infusion catheter for the treatment of embolic stroke. AJNR Am J Neuroradiol 2003;24:534–38 [PMC free article] [PubMed] [Google Scholar]
- 5.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke [published erratum appears in Stroke 2003;34:2774]. Stroke 2003;34:e109–37 [DOI] [PubMed] [Google Scholar]
- 6.Khatri P, Neff J, Broderick JP, et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke 2005;36:2400–03 [DOI] [PubMed] [Google Scholar]
- 7.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–11 [DOI] [PubMed] [Google Scholar]
- 8.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–38 [DOI] [PubMed] [Google Scholar]
- 9.Nakano S, Yokogami K, Ohta H, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 1998;19:767–72 [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano S, Yokogami K, Ohta H, et al. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion: report of two cases. Angiology 1997;6:254–56 [PMC free article] [PubMed] [Google Scholar]
- 11.Purdy PD, Devous MD, Unwin DH, et al. Angioplasty of an atherosclerotic middle cerebral artery associated with improvement in regional cerebral blood flow. AJNR Am J Neuroradiol 1990;11:878–80 [PMC free article] [PubMed] [Google Scholar]
- 12.Ringer AJ, Qureshi AI, Fessler RD, et al. Angioplasty of intracranial occlusion resistant to thrombolysis in acute ischemic stroke. Neurosurgery 2001;48:1282–88; discussion 1288–90 [DOI] [PubMed] [Google Scholar]
- 13.Tsai FY, Berberian B, Matovich V, et al. Percutaneous transluminal angioplasty adjunct to thrombolysis for acute middle cerebral artery rethrombosis. AJNR Am J Neuroradiol 1994;15:1823–29 [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano S, Iseda T, Yoneyama T, et al. Intravenous low-dose native tissue plasminogen activator for distal embolism in the middle cerebral artery divisions or branches: a pilot study. Neurosurgery 2000;46:853–58; discussion 858–59 [DOI] [PubMed] [Google Scholar]
- 15.Yoneyama T, Sakano S, Kawano H, et al. Combined direct percutaneous transluminal angioplasty and low-dose native tissue plasminogen activator therapy for acute embolic middle cerebral artery trunk occlusion. AJNR Am J Neuroradiol 2002;23:277–81 [PMC free article] [PubMed] [Google Scholar]
- 16.Barnwell SL, Clark WM, Nguyen TT, et al. Safety and efficacy of delayed intraarterial urokinase therapy with mechanical clot disruption for thromboembolic stroke. AJNR Am J Neuroradiol 1994;15:1817–22 [PMC free article] [PubMed] [Google Scholar]
- 17.Noser EA, Shaltoni HM, Hall CE, et al. Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke 2005;36:292–96 [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Nedeltchev K, Mattle HP, et al. Intra-arterial thrombolysis in 24 consecutive patients with internal carotid artery T occlusions. J Neurol Neurosurg Psychiatry 2003;74:739–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaidat OO, Suarez JI, Santillan C, et al. Response to intra-arterial and combined intravenous and intra-arterial thrombolytic therapy in patients with distal internal carotid artery occlusion. Stroke 2002;33:1821–26 [DOI] [PubMed] [Google Scholar]
- 20.Christoforidis GA, Mohammad Y, Kehagias D, et al. Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 2005;26:1789–97 [PMC free article] [PubMed] [Google Scholar]
- 21.Mori E, Tabuchi M, Yoshida T, et al. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988;19:802–12 [DOI] [PubMed] [Google Scholar]
- 22.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–87 [DOI] [PubMed] [Google Scholar]
- 23.Wardlaw JM, Warlow CP, Counsell C. Systemic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet 1997;350:607–14 [DOI] [PubMed] [Google Scholar]
- 24.Gönner F, Remonda L, Mattle, H, et al. Local intra-arterial thrombolysis in acute ischemic stroke. Stroke 1998;29:1894–900 [DOI] [PubMed] [Google Scholar]
- 25.Donnan GA, Davis SM, Chambers BR, et al. Streptokinase in acute ischemic stroke with relationship to time of administration: Australian Streptokinase (ASK) Trial Study Group. JAMA 1996;276:961–66. [PubMed] [Google Scholar]
- 26.von Kummer R, Holle R, Rosin L, et al. Does arterial recanalization improve outcome in carotid territory stroke? Stroke 1995;26:581–87 [DOI] [PubMed] [Google Scholar]
- 27.Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology 2003;45:11–18 [DOI] [PubMed] [Google Scholar]
- 28.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol 1998;55:1475–82 [DOI] [PubMed] [Google Scholar]
- 29.Bisschops RH, Klijn CJ, Kappelle LJ, et al. Collateral flow and ischemic brain lesions in patients with unilateral carotid artery occlusion. Neurology 2003;60:1435–41 [DOI] [PubMed] [Google Scholar]
- 30.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol 1993;14:3–13 [PMC free article] [PubMed] [Google Scholar]
- 31.Freitag HJ, Becker VU, Thie A, et al. Lys-plasminogen as an adjunct to local intra-arterial fibrinolysis for carotid territory stroke: laboratory and clinical findings. Neuroradiology 1996;38:181–85 [DOI] [PubMed] [Google Scholar]