Abstract
Background
Glioblastoma (GB) is the most common malignant brain tumor with a dismal prognosis despite standard of care (SOC). Here we used a network meta-analysis on treatments from randomized control trials (RCTs) to assess the effect on overall survival (OS) and progression-free survival (PFS) beyond the SOC.
Methods
We included RCTs that investigated the addition of a new treatment to the SOC in patients with newly diagnosed GB. Our primary outcome was OS, with secondary outcomes including PFS and adverse reactions. Hazard ratio (HR) and its 95% confidence interval (CI) regarding OS and PFS were extracted from each paper. We utilized a frequentist network meta-analysis. We planned a subgroup analysis based on O6-methylguanine-DNA methyl-transferase (MGMT) status. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Results
Twenty-one studies were included representing a total of 7403 patients with GB. There was significant heterogeneity among studies impacting important factors such as timing of randomization and sample size. A confidence analysis on the network meta-analysis results revealed a score of low or very low for all treatment comparisons, across subgroups. Allowing for the heterogeneity within the study population, alkylating nitrosoureas (Lomustine and ACNU) and tumor-treating field improved both OS (HR = 0.53, 95% CI 0.33–0.84 and HR = 0.63 95% CI 0.42–0.94, respectively) and PFS (HR = 0.88, 95% CI 0.77–1.00 and HR = 0.63 95% CI 0.52–0.76, respectively).
Conclusions
Our analysis highlights the numerous studies performed on newly diagnosed GB, with no proven consensus treatment that is superior to the current SOC. Intertrial heterogeneity raises the need for better standardization in neuro-oncology studies.
Keywords: glioblastoma, network meta-analysis, randomized control trial, standard of care, temozolomide
Key Points.
Our analysis highlights the numerous studies performed on newly diagnosed glioblastoma, with no proven consensus treatment that is superior to the current standard of care.
Intertrial heterogeneity precludes drawing strong conclusions, and confidence analysis was low.
Importance of the Study.
Glioblastoma remains a challenging clinical entity to treat. Few advances have been made since the advent of the current standard of care—this is despite a myriad of clinical trials performed. Direct head-to-head comparisons of these therapies would be difficult and resource-intensive. We employed a network meta-analysis to perform direct and indirect comparisons of therapies and their effect on treatment outcomes. Our analysis highlights the numerous studies performed on newly diagnosed glioblastoma, with no proven consensus treatment that is superior to the current treatment paradigm. Intertrial heterogeneity precludes drawing strong conclusions, and confidence analysis of the meta-analysis was low. Our study presents a novel method for analyzing clinical trials, identifies the need for greater standardization across studies, and highlights some areas which may be worthwhile to pursue in future research studies.
Glioblastoma (GB) is the most common primary adult brain tumor and is also one of the most aggressive diseases that afflict humans.1 The current standard of care (SOC) commonly referred to as the “Stupp Protocol” includes maximal safe surgical cytoreduction followed by daily temozolomide (TMZ) and concurrent radiotherapy (60 Gy in 30 fractions), with subsequent adjuvant TMZ for 6 months.2 This approach was first introduced in 2005 and prolonged median survival by 2.5 months with a 2-year survival rate of 27% compared to 10% with radiation alone after maximal safe resection. Despite maximal therapies, the median overall survival (OS) of GB patients is 15 months, with 5-year survival rates of less than 5%.2 A subset of patients with favorable molecular profiles, including O6-methylguanine-DNA methyl-transferase (MGMT) methylation and isocitrate dehydrogenase (IDH)1/2 mutations, benefit more from this protocol. Patients with these molecular changes can have their median OS extended up to about 30 months on average.3 In the post-Stupp protocol era, there is a lack of consensus on new proven clinical treatments despite an abundance of preclinical and clinical research.
Despite the abundance of research on this area, there has yet to be a consensus, evidence-based treatment that has been effective in clinical settings beyond Stupp protocol.4 As such, patients with GB continue to portend bleak prognoses. Challenges in deriving novel treatments stem both from biological heterogeneities intrinsic to the disease and wide-ranging clinical differences in terms of patient status, treatment standards, and reporting standards.
To help illustrate the current landscape of GB treatment, we performed a systematic review and network meta-analysis comparing the efficacy of differential treatment regimens in phase 2 or 3 randomized control trials (RCTs) for newly diagnosed GB (primary or secondary), where the control arm included the use of the Stupp protocol. Traditional meta-analysis allows comparison between only 2 treatment arms. On the contrary, network meta-analysis allows for the comparison of more than 2 different treatment arms for a given pathology. Given the abundance of different trials on GB with different arms, the application of a network meta-analysis to our research question was ideal, with the aim of viewing an overall picture of advancements in the field, and compares the currently available treatment arms.5,6
Methods
We conducted our systematic review and network meta-analysis based on a predefined protocol in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension statement for reporting on network meta-analyses.7 Databases including MEDLINE (PubMed and Ovid), Embase, and Web of Science were searched until July 1, 2019. We used, in relevant combinations, keywords and MeSH (Medical Subject Heading) terms pertaining to the patient population disease (high-grade glioma, GB) and clinical trial. Abstracts were screened for potential inclusion criteria by both primary authors, and subsequently, the full text of the articles of interest was reviewed. We extracted the following variables from each included paper: age, sex, IDH status, MGMT status, definition of MGMT methylation, definition of progression, median OS, median progression-free survival (PFS), hazard ratios (HRs) and its 95% confidence intervals (CIs), extent of resection, and frequency of adverse events (AEs).
Our inclusion criteria included RCTs of phase 2 or 3 studies with 20 or more patients in each treatment arm, patients with GB (astrocytoma grade IV) without prior treatments who have undergone maximal safe resection prior to adjuvant therapy. The control group in included studies must have received at minimum the SOC, that is, maximal safe resection with Stupp protocol. Articles were excluded if results on GB patients could not be separated from non-GB patients included in trials and if data on primary and secondary outcomes were not available.
Quality assessment of the included studies was done using Cochrane’s Risk of Bias Tool for randomized trials.8 This previously validated tool is designed to assess the quality and risk of bias for RCTs.
Our primary outcome was OS, with secondary outcome including PFS and adverse reactions. We only included studies that reported HR of comparison between arms of the trial for OS and PFS. If we were not able to extract the HR for PFS or OS from the published study or obtain that from the corresponding author, the study was excluded.
Statistical Analysis
In this study, we took the approach that synthesizes metrics of both direct and indirect comparisons to refine and generate estimates of all possible pair-wise comparisons within a network.9–11 For comparative efficacy analysis, we utilized a frequentist network meta-analysis, an extension of the classic pair-wise meta-analysis, to compare multiple different treatments across trials on a common comparator in a single unified analysis. Estimates of treatment effect (HR) via direct comparisons were made between treatment groups within a single trial and an indirect comparison of treatment effect between different trials with a common comparator (eg, Stupp protocol) was estimated from the direct treatment effects. Multiple indirect comparisons were then made for each treatment modality. When both direct and indirect evidence of comparison between treatment modalities was available, the treatment effect was synthesized together to yield a network treatment effect. A single combined ranking of treatments was then produced based on Rücker and Schwarzer method.12
We assessed heterogeneity using Cochran’s Q statistics. A P value of .1 was considered significant heterogeneity. In case of heterogeneity within a pooled data set between studies, a random effect model was used. A fix effect model was used when pooled data were homogenous. We planned a subgroup analysis based on the MGMT status in advance. A 2-way P value of less than .05 was considered statistically significant. R software version R 3.6.3 was used for all analyses.
To assess the confidence in the results of the network meta-analysis, we utilized a previously described method, the Confidence in Network Meta-Analysis (CINeMA) framework and software.13,14 This framework incorporates 6 domains to determine the level of confidence in the network meta-analysis results: (1) within‐study bias, (2) reporting bias, (3) indirectness, (4) imprecision, (5) heterogeneity, and (6) incoherence.
Results
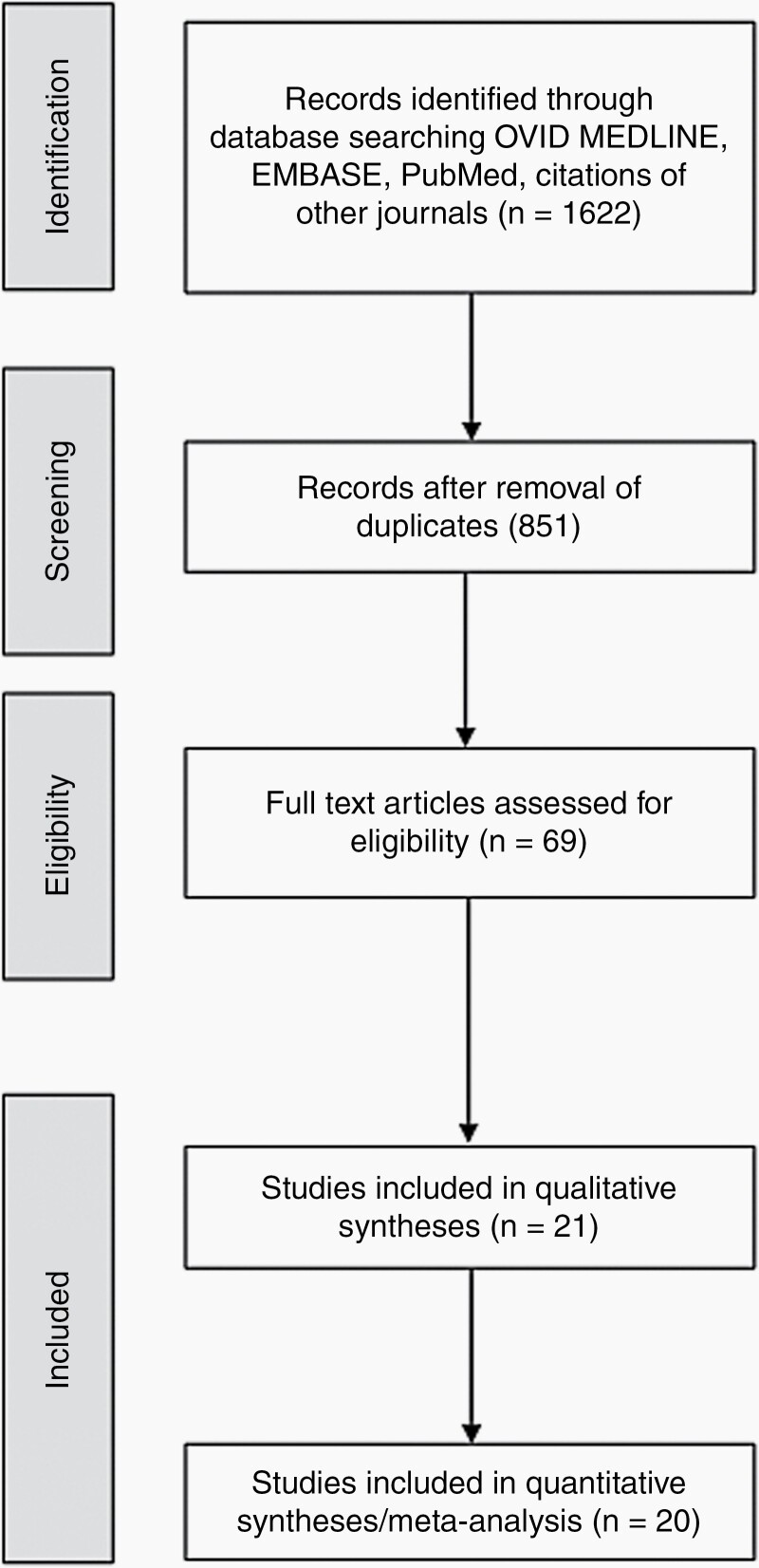
Our literature search included 1622 initial results. After removal of duplicates and abstract screening, 69 papers underwent full-text review. Twenty-one studies were included representing a total of 7403 patients with GB. A study flow diagram is shown in Figure 1, and study characteristics are summarized in Table 1.15–36 There were 3747 patients in the treatment arms and 2981 patients in the control arm. Thirty-five percent of patients in the treatment arms and 38.7% of patients in the control arm had MGMT promoter methylated. IDH mutation was detected in 7.25% of patients in treatment arms and 5.7% of patients in the control arm, respectively.
Figure 1.
Study flow chart.
Table 1.
Literature Review Results of Randomized Control Trials on Newly Diagnosed GB
| Study | Treatment | Mechanism | No. Patients | Age | Randomization after SOC | %GTR | %MGMT Methylated | % IDH Mutant | Median OS (months) | OS HR | Median PFS (months) | PFS HR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wen et al. 2019 | ICT-107 | Dendritic cell vaccine | 75 | 59 | Yes | 71.6 | 34.6 | NR | 17 | 0.87 | 11.2 | 0.57 |
| Control | 42 | 59.8 | 74.4 | 41.9 | NR | 15 | 9 | |||||
| Herrlinger et al. 2019 | Lomustine + TMZ | Alkylating nitrosourea | 66 | 56 | No | 59.0 | 1000 | 5.0 | 48.1 | 0.6 | 16.7 | 0.91 |
| Control | 63 | 59 | 63.0 | 100 | 8.0 | 31.4 | 16.7 | |||||
| Yang et al. 2018 | ACNU | Alkylating nitrosourea | 44 | NR | No | NR | 54.5 | 20.5 | 18.5 | 0.453 | 8.8 | 0.496 |
| Control | 27 | NR | NR | 66.7 | 14.8 | 16 | 7 | |||||
| Wakabayashi et al. 2018 | Interferon-β | MGMT downregulator | 59 | 61 | No | 55.9 | NR | 0 | 24 | 1.0 | 10.1 | 1.25 |
| Control | 63 | 61 | 49.2 | NR | 0 | 20.3 | 8.5 | |||||
| Mallick et al. 2018 | Hypofractionated RT | RT | 45 | 45 | No | 62.8 | NR | NR | 18.1 | 1.52 | 13.1 | |
| Control | 38 | 37.2 | NR | NR | 25.1 | 14.1 | ||||||
| Elinzano et al. 2018 | PPX | Radiosensitizer | 41 | 62 | No | 90.5 | 0 | NR | 16 | 1.44 | 9 | 1.1 |
| Contol | 18 | 62 | 100 | 0 | NR | 14.8 | 9.5 | |||||
| Chinnaiyan et al. 2018 | Everolimus | mTOR inhibitor | 88 | NR | No | 51.1 | NR | NR | 16.5 | 1.67 | 13.8 | 1.15 |
| Control | 83 | NR | 57.8 | NR | NR | 21.2 | 10.2 | |||||
| Buchroithner et al. 2018 | Dendritic cell vaccine | Dendritic cell vaccine | 34 | 54.6 | Yes | 71.0 | 35.0 | NR | 18.8 | 0.99 | — | — |
| Control | 42 | 54 | 83.0 | 35.0 | NR | 18.9 | — | |||||
| Weller et al. 2017 | Rindopepimut | Anti-EGFR | 371 | 59 | Yes | NR | 33.0 | NR | 20.1 | 1.01 | 8 | 1.01 |
| Control | 374 | 58 | NR | 35.0 | NR | 20 | 7.4 | |||||
| Ursu et al. 2017 | IC CpG-ODN | TLR9 modulation | 42 | 62 | No | 79.0 | 26.0 | 12.0 | 17 | 1.2 | 9 | 1.2 |
| Control | 39 | 59 | 65.0 | 31.0 | 12.0 | 18 | 9 | |||||
| Stupp et al. 2017 | Tumor-treating fields | Anti-mitotic | 812 | 56 | Yes | 53.0 | 36.0 | 7.0 | 20.9 | 0.63 | 6.7 | 0.63 |
| Control | 401 | 57 | 54.0 | 42.0 | 5.0 | 16 | 4.0 | |||||
| Kong et al. 2017 | CIK immunotherapy | Cytotoxic lymphocytes | 91 | 55 | No | 48.4 | NR | NR | 22.47 | 0.693 | 8.1 | 0.745 |
| Control | 89 | 54 | 53.9 | NR | NR | 16.8 | 5.4 | |||||
| Wick et al. 2016 | Temsirolimus | mTOR inhibitor | 53 | 54.9 | No | 94.6 | 0 | NR | 14.78 | 1.16 | 5.36 | 1.26 |
| Control | — | 55 | 57.7 | 98.2 | 0 | NR | 16.0 | 5.45 | ||||
| Herrlinger et al. 2016 | Bevacizumab/Irinotecan | Anti-VEGF | 116 | 56 | No | 50.0 | 0 | NR | 17.5 | 1.02 | 9.7 | 0.57 |
| Control | — | 54 | 56 | 46.3 | 0 | NR | 16.6 | 6.0 | ||||
| Westphal et al. 2015 | Nimotuzumab | Anti-EGFR | 71 | 55 | No | 43.7 | 21.1 | NR | 22.3 | 0.862 | 7.7 | 0.953 |
| Control | — | 71 | 56 | 42.3 | 22.5 | NR | 19.6 | 5.8 | ||||
| Penas-Prado et al. 2015 | Thalidomide | Anti-angiogenesis | 21 | NR | Yes | 61.9 | NR | NR | 11.7 | 2.2 | 6.5 | 2 |
| Isotretinoin | Differentiation inhibitor | 21 | NR | 77.8 | NR | NR | 18.1 | 1.2 | 13.4 | 1 | ||
| Celecoxib | COX-2 inhibitor | 18 | NR | 76.2 | NR | NR | 17.4 | 1.7 | 7.7 | 1.6 | ||
| Thalidomide/Isotretinoin | — | 21 | NR | 45.0 | NR | NR | 23.1 | 1.1 | 11.6 | 1.1 | ||
| Thalidomide/Celecoxib | — | 17 | NR | 58.8 | NR | NR | 17.9 | 1.3 | 6.2 | 1.4 | ||
| Isotretinoin/Celecoxib | — | 20 | NR | −57.1 | NR | NR | 20.2 | 1.1 | 7.9 | 1.2 | ||
| Thalidomide/Celecoxib/Isotretinoin | — | 15 | NR | 93.3 | NR | NR | 18.5 | 1.4 | 5.8 | 1.7 | ||
| Control | — | 22 | NR | 50.0 | NR | NR | 21.2 | 10.5 | ||||
| Nabors et al. 2015 | Cilengitide | Integrin inhibitor | 88 | 55.6 | No | 50.0 | 0 | NR | 16.3 | 0.686 | 5.6 | 0.822 |
| Intensive Cilengitide | 88 | 56 | 52.3 | 0 | NR | 14.5 | 0.858 | 5.9 | 0.794 | |||
| Control | — | 89 | 57.7 | 51.7 | 0 | NR | 13.4 | 4.1 | ||||
| Stupp et al. 2014 | Cilengitide | Integrin inhibitor | 272 | 58 | No | 49.0 | 100 | NR | 26.32 | 1.02 | 10.6 | 0.92 |
| Control | — | 273 | 58 | 50.0 | 100 | NR | 26.3 | 7.9 | ||||
| Gilbert et al. 2014 | Adjuvant Bevacizumab | Anti-VEGF | 260 | 59 | Yes | 63.0 | 29.0 | NR | 15.7 | 1.13 | 10.7 | 0.79 |
| Control | — | 248 | 57 | 42.3 | 27.4 | NR | 16.1 | 7.3 | ||||
| Chinot et al. 2014 | Concomitant/adjuvant Bevacizumab | Anti-VEGF | 458 | 57 | No | 41.0 | 25.5 | NR | 16.8 | 0.88 | 10.6 | 0.64 |
| Control | — | 463 | 56 | 42.3 | 27.4 | NR | 16.7 | 6.2 | ||||
| Gilbert et al. 2013 | Dose-dense TMZ | Alkylating nitrosurea | 422 | NR | No | 52.0 | 29.0 | NR | 14.9 | 1.03 | 6.7 | 0.87 |
| Control | — | 411 | NR | 56.0 | 30.0 | NR | 16.6 | 5.5 | ||||
| Cho et al. 2012 | Dendritic cell vaccine | Dendritic cell vaccine | 18 | NR | Yes | 77.8 | 56.3 | NR | 31.9 | 0.23 | 8.5 | |
| Control | — | 16 | NR | 68.8 | 55.6 | NR | 15 | 8 |
NR, not reported.
The search resulted in 27 different treatment arms, with very little overlap between studies. Two studies focused on dendritic cell vaccines.22,30 Cilengitide treatment was present across multiple studies.17,33 Bevacizumab was studied in multiple trials, but varied in its use with irinotecan,16 as concomitant adjuvant treatment,36 or adjuvant treatment alone.34 The Lomustine–TMZ study included only MGMT methylated patients,15 and Bevacizumab/Irininotecan,16 Temsirolimos, and paclitaxel poliglumex26 trials included only unmethylated patients. For the 2 studies on Cilengitide, one included only MGMT methylated,17 and the other included only MGMT unmethylated patients.33 The mechanisms of all medications are listed in Table 1. Non-combined data are shown in Supplementary Figure 1.
As expected, there was significant intertrial variability on reported patient demographic and molecular results, and trials also differed widely between the timing of randomization and enrollment of patients either before or after the completion of SOC (Table 1).
Quality of Evidence
The overall risk of bias based on the Cochrane Collaboration tool was medium for 3 of the studies22,31,32 and low risk in the remainder. Detailed quality assessment results are available in Supplementary Figure 2.
Survival Outcomes
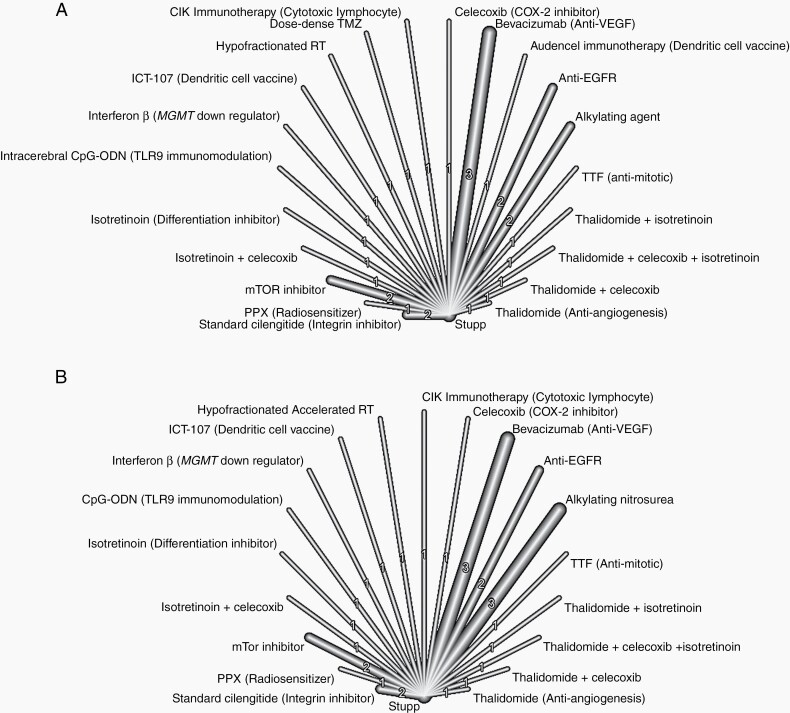
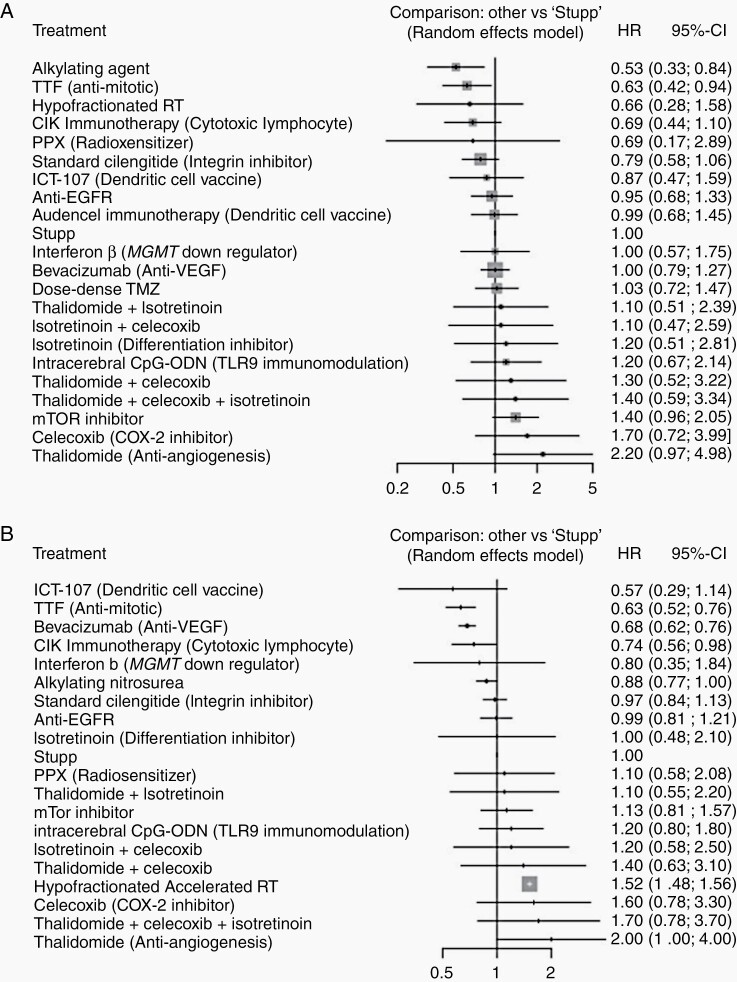
All 21 studies were included in an analysis of OS. A random effect model was used. Two cilengitide papers, one on MGMT methylated and one on MGMT unmethylated patients, were combined.17,33 Everolimus and Temsirolimus were grouped together (mTOR inhibitors). Rindopepimut and Nimotuzumab were grouped together (anti-EGFR). Various Bevacizumab treatments were also combined together (Bevacizumab). Lomustine and ACNU were combined into alkylating nitrosourea. A random effect model was used (network graph, Figure 2A). The forest plot for studies included in the OS is shown in Figure 3A and Supplementary Figure 5A.
Figure 2.
Combined network graph for (A) OS and (B) PFS.
Figure 3.
Forest plots for included studies in (A) OS and (B) PFS.
Alkylating nitrosourea (HR = 0.53, 95% CI 0.33–0.84), and tumor-treating fields (TTFs; HR = 0.63, 95% CI 0.42–0.94), showed statistically significant improvement in OS when compared to SOC. CIK immunotherapy, cytotoxic lymphocyte (HR = 0.69, 95% CI 0.44–1.10), and cilengitide (HR = 0.79, 95% CI 0.58–1.06) showed a trend toward significant alkylating agents (P = .91) followed by TTF (P = .84) and CIK immunotherapy (P = .78) ranked the first 3 treatments that improved OS (Figure 4A).
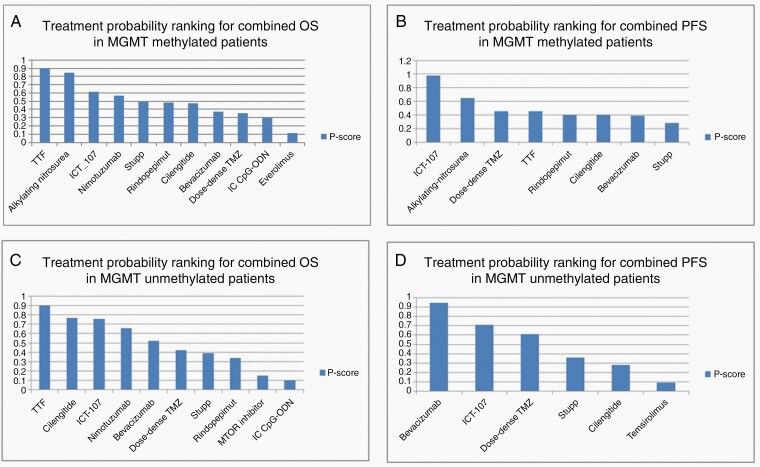
Figure 4.
Treatment probability rankings for (A) OS in MGMT methylated patients (B) PFS in MGMT methylated patients (C) OS in MGMT unmethylated patients, and (D) PFS in MGMT unmethylated patients.
The CINeMA analysis is shown in Supplementary Figure 3. The confidence rating for all direct and indirect comparisons was very low.
Progression-Free Survival
Twenty studies were included in the analysis of PFS. A random effect model was used (network graph, Figure 2B). There were 3279 patients in the treatment arms and 2628 patients in the control arm. The rate of MGMT promoter methylation was 25.9% in treatment arms and 29.9% in control arms.
The definition of PFS used in each trial is summarized in Table 1. Grouping of studies together was done similar to OS analysis. The forest plot for studies included in PFS is shown in Figure 3B. Bevacizumab (HR = 0.68, 95% CI 0.62–0.76), TTF (HR = 0.63, 95% CI 0.52–0.76), and CIK immunotherapy (HR = 0.74, 95% CI 0.56–0.98) showed statistically significant PFS outcomes when compared with Stupp protocol. Alkylating agents showed a trend (HR = 0.88, 95% CI 0.77–1.00). ICT-107 (dendritic cell vaccine) showed a nonsignificant trend toward improving PFS (HR = 0.57, 95% CI 0.29–1.14). The first 3 ranked treatments in order were TTF (P = .91), ICT-107 (P = .89), and Bevacizumab (P = .86, Supplementary Figure 5B).
The CINeMA analysis is shown in Supplementary Figure 4. The confidence rating for all direct and indirect comparisons was very low, except for one treatment rated as low.
OS in MGMT Methylated Patients
We performed a subgroup analysis of our primary and secondary outcomes on patients with known MGMT promoter methylation. Twelve studies were included in the analysis of OS with MGMT promoter methylation status accounting for 1300 patients in the treatment arms and 1145 patients in the control arms. Treatment arms included TTF, ACNU, Lomustine, ICT-107, Nimotuzumab, concomitant/adjuvant Bevacizumab, rindopepimut, cilengitide, dose-dense TMZ, intracerebral CpG-ODN, and everolimus (for non-combined analysis see Supplementary Figure 6A).
The method of assessing MGMT promoter methylation in these studies included real-time PCR using the ratio of MGMT to the βactin reference gene (ACTB) greater than 2 in 5 studies,15,17,18,34,35 the methylated MGMT/COL2A1 ratio greater than 0 in one study,20 nested PCR in one study,37 pyrosequencing in one study,19 and not specified in 4 trials.22,23,27,36
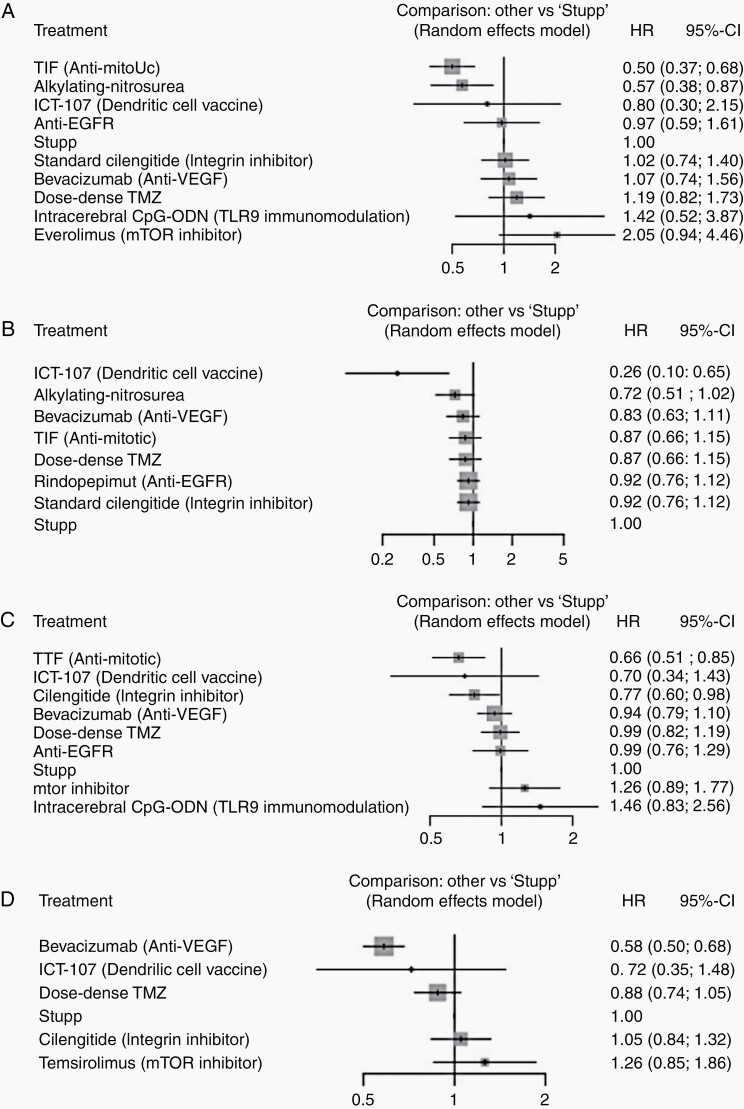
For this analysis, ACNU and Lomustine were grouped together (alkylating nitrosourea).15,23 Two Bevacizumab studies were grouped together (Bevacizumab).34,36 Nimotuzumab and Rindopepimut were combined (anti-EGFR); this is shown in Supplementary Figure 6B. A random effect model was used to pool the data. TTF (HR = 0.5, 95% CI 0.37–0.68) and alkylating nitrosourea (HR = 0.57, 95% CI 0.38–0.87) significantly improved OS when added to SOC (Figure 5A). In patients with MGMT promoter methylation, TTF (P = .94) and alkylating nitrosoureas (P = .87) ranked first and second in improving OS. This is shown in Figure 4A.
Figure 5.
Subgroup forest plots for (A) OS in MGMT methylated patients, (B) PFS in MGMT methylated patients, (C) OS in MGMT unmethylated patients, and (D) PFS in MGMT unmethylated patients.
The CINeMA analysis is shown in Supplementary Figure 7. The confidence rating for all direct and indirect comparisons was very low.
PFS in MGMT Methylated Patients
Nine studies were included in the analysis of PFS with MGMT promoter methylation status accounting for 848 patients in the treatment arms and 785 patients in the control arms. Response Assessment in Neuro-Oncology (RANO) criteria were used to define PFS in 3 studies,15,22,37 Macdonald criteria were used to define PFS in 5 studies,17,34,35,38 and PFS definition did not use either RANO or Macdonald criteria in one study.23
The method of assessing MGMT promoter methylation in these studies included real-time PCR using the ratio of MGMT to the βactin reference gene (ACTB) greater than 2 in 5 studies,15,17,18,34,35 nested PCR in one study,37 pyrosequencing in one study,19 and not specified in 3 trials.22,23,27,36 Methylation definition is summarized in Supplementary Table 1.
Treatments included in the analysis of this subgroup were ICT-107, ACNU, concomitant/adjuvant bevacizumab, dose-dense TMZ, TTF, Lomustine, standard cilengitide, and rindopepimut (for non-combined analysis see Supplementary Figure 8A). For this analysis, ACNU and Lomustine were grouped together (alkylating nitrosourea). Two Bevacizumab studies were grouped together (Bevacizumab). A fixed effect model was used to pool the data (Supplementary Figure 8B).
ICT-107 significantly improved PFS (HR = 0.26, 95% CI 0.10–0.65) when compared with Stupp protocol. ACNU also showed a trend toward improving PFS (HR = 0.72, 95% 0.51–1.02). This is shown in Figure 5C. In patients with MGMT promoter methylation, ICT-107 (P = .99) ranked first followed by alkylating nitrosoureas (combined analysis of ACNU and Lomustine, P = .72, Supplementary Figure 4B) in improving PFS.
The CINeMA analysis is shown in Supplementary Figure 9. The confidence rating for all direct and indirect comparisons was very low.
OS in MGMT Unmethylated Patients
Thirteen studies were included in a subgroup analysis of OS in MGMT unmethylated patients, accounting for 2436 patients in the treatment arms and 1819 patients in the control arms. Treatments included in this analysis were TTF, standard cilengitide, ICT-107, nimotuzumab, intensive cilengitide, concomitant/adjuvant bevacizumab, bevacizumab/irinotecan, dose-dense TMZ, rindopepimut, temsirolimus, intracerebral CpG-ODN, and everolimus (for non-combined analysis see Supplementary Figure 10A).16,18–20,22,27,31,33,34,36,37
Temsirolimus and Everolimus were grouped together (MTOR inhibitor),27,31 3 Bevacizumab studies were grouped together (Bevacizumab),16,34,36 2 Cilengitide HRs from the same study were combined into one HR.33 Rindopepimut and nimotuzumab were grouped together (anti-EGFR). This is shown in Supplementary Figure 10B. A random effect model was used to pool the data.
In patients with nonmethylated MGMT promoter status, TTF (HR = 0.66, 95% CI 0.51–0.85) and standard dose cilengitide (HR = 0.77, 95% CI 0.60–0.98) significantly improved survival (Figure 5B). In patients without MGMT promoter methylation, TTF (P = .91) ranked first followed by standard dose cilengitide (P = .79, Figure 4C) in improving OS.
The CINeMA analysis is shown in Supplementary Figure 11. The confidence rating for all direct and indirect comparisons was very low.
PFS in MGMT Unmethylated Patients
Seven studies were included in the PFS analysis accounting for 1219 patients in the treatment arms and 1026 patients in the control arms. The RANO criteria were used to define PFS in one study,22 while the remaining studies used Macdonald criteria to define PFS.16,31,33–36 Treatments included in this analysis of PFS included concomitant/adjuvant bevacizumab, ICT-107, cilengitide, dose-dense TMZ, and temsirolimus (for non-combined analysis see Supplementary Figure 12A and B).22,31,33,35,36
Three Bevacizumab studies were analyzed together (Bevacizumab),16,34,36 2 Cilengitide HRs from the same study were combined into one HR.33 There was no data on TTF for this subgroup. A random effect model was used to pool the data.
In patients without MGMT promoter methylation, bevacizumab significantly improved PFS when compared to control (HR = 0.58, 95% CI 0.50–0.68, Figure 5D). In patients with unmethylated MGMT promoter status, bevacizumab had the highest probability of improving PFS (P = .94, Figure 4D).
The CINeMA analysis is shown in Supplementary Figure 13. The confidence rating for all direct and indirect comparisons was rated as very low.
Safety Analysis
Meaningful, pooled analysis of AEs was not possible as a result of the heterogeneity within the reported variables. We tabulated the average frequency of AE’s different grades for each treatment arm. In general, AEs happened more frequently for every AE grade in the treatment arm as compared to the control arm (Supplementary Table 2).
Discussion
In the post-Stupp protocol era, there is a lack of consensus on new proven clinical treatments despite an abundance of preclinical and clinical research. Heterogeneity of the disease and a range of different methodologies in assessing the response rate to treatment add to the complexity of deriving consensus regarding a unified treatment paradigm. We focused our analysis on RCTs to perform a network meta-analysis to determine the efficacy of treatments in patients with newly diagnosed GB in relation to each other.
Our analysis depicts the wide range of treatment modalities that have been studied in the treatment of GB and highlights the lack of a proven, consensus treatment that is superior to the current SOC. It is important to view the results of our analysis bearing in mind its methodological intentions and limitations. The treatments identified via a network meta-analysis may not be the objective best treatment, but rather are determined to be the best treatment based on the included studies. In an attempt to quantify the confidence of the network meta-analysis results, we used a previously described methodology.13,14 Comparisons for all primary and secondary endpoints, and across all subgroups, received a score of very low or low. This highlights the exploratory nature of our study.
This low confidence score helps to highlight an important point from our study which is the heterogeneity that exists across neuro-oncology trials in vital factors, such as the timing of randomization either before or after patients receive SOC. Randomization after receiving SOC provides a survival net for study patients, but also introduces survival bias, as patients with the more significant disease may not survive long enough to reach randomization. This prevents generalizability across different patient and study populations, especially in analytic synthesis such as a meta-analysis. For example, in our analysis, the addition of TTF38 to the SOC ranked high in improving OS in all subgroups and improved overall PFS but this outcome may be influenced by the fact that randomization in this trial occurred after delivery of SOC. Furthermore, an additional perspective is that data from the TTF trials did not include information on PFS in patients without MGMT promoter methylation. As many treatment options continue to emerge for newly diagnosed GB, the need for consensus and homogeneity is clear. Standardizing clinical trials, such as baseline genetic evaluation of the tumor, time and type of randomization, and definition of outcome, will help to better determine treatment value and impact.
Alkylating agents (ACNU and Lomustine) improved OS for all newly diagnosed GB patients and on those with MGMT methylation. This statistical result is likely driven by the small sample size in the ACNU trial. Additional trials studying ACNU and other alkylating agents are needed to better understand the effect these drugs can have either on their own or in combination with SOC. Of note, combined with TMZ these drugs showed a favorable AE profile when compared with control.
While the field recognizes the value of MGMT methylation, and MGMT methylation status often directs clinical decision making, not all trials have uniform reporting of MGMT or have taken into consideration this factor for stratification. A number of the trials included a study population entirely of patients with either MGMT methylated15,17 or unmethylated status.16,26,31,33 When these studies are included into the entire study GB cohort, the specific treatment effect may be under- or overestimated depending on the direction of its effect. For example, bevacizumab improved PFS in MGMT unmethylated patients, but had no effect on MGMT methylated patients. In the overall study population, bevacizumab appeared to improve PFS, likely due to the fact that approximately 75% of patients treated with bevacizumab had MGMT unmethylated status.
In addition to MGMT methylation status, analysis of GB data by The Cancer Genome Atlas Research Network shows that the most frequently altered genes include amplification of EGFR, mutation of TP53, phosphatidylinositol-4,5-bisphosphate 3-kinase A (PIK3CA), PTEN, IDH1, RB1, and TERT promoter, and deletions of PTEN, CDKN2A/B, and MGMT.39 Notably, CDKN2 mutations have been shown to have important prognostic value in IDH-mutant gliomas.40 Similar to how the treatment effect of TMZ is impacted by MGMT promoter methylation status, the effect of current treatments for GB, including those reviewed here in our analysis, may be impacted by genetic mutations and cellular heterogeneity. Of note, there was insufficient data to perform subgroup analysis on IDH mutation.
Our analysis showed that certain agents prolong PFS without prolonging OS, raising the question whether these drugs are masking clinical progression; an example is Bevacizumab (anti-VEGF). VEGF inhibitors have been shown to cause a decrease in cerebral vascular permeability and a subsequent decrease in cerebral edema.41 Clinical progression may be masked if only T1-weighted sequences are used in the evaluation of progression, such as with the Macdonald criteria.42 This has now widely been replaced by the newer RANO criteria,43 with the most significant difference being that the RANO factors in T2-weighted changes (FLAIR) when assessing progression. It is conceivable that anti-VEGF agents may appear to have an effect on PFS depending on the criteria used to assess progression. In fact, in our analysis the studies with anti-VEGF treatments primarily used the Macdonald criteria—these outcomes may have been different using a different set of diagnostic criteria.
Direct comparison between 2 or more modalities is resource-intensive. Our analysis, by mainly using indirect comparisons between treatments, provides an overall estimate of quantitative effects and highlights the existing landscape in the treatment of GB. In this analysis, some treatments (eg, TTF or alkylating nitrosoureas) appeared to improve OS or PFS, either in specific subgroups or in the study population as a whole; however, as outlined above, there are some methodological drawbacks that need to be addressed in future studies. Moreover, as the number of trials assessing those treatments with positive effects increases, the true impact of these additional treatments to SOC on patients with GB will be elucidated. An important consideration when viewing this meta-analysis is to understand that while agents may have similar biological targets resulting in grouping together for network meta-analysis, they may have differing biochemical properties that may affect pharmacokinetics or pharmacodynamics including access across the blood–brain barrier. This reinforces that our study is meant to provide a broad overview of the current landscape in GB therapy, rather than focusing on individual treatment results.
Conclusions
We present the first study using a network meta-analysis to examine RCT data on the initial treatment of GB in the post-Stupp protocol era. Our analysis highlights the numerous studies performed on newly diagnosed GB, with no proven consensus treatment that is superior to the current SOC. Intertrial heterogeneity raises the need for better standardization in neuro-oncology studies.
Supplementary Material
Funding
There are no funding supports to declare for this work.
Conflict of interest statement. The authors have no conflicts of interest to declare.
Authorship Statement Conception and design: S.T., V.Y., and G.Z.; development of methodology: S.T., V.Y., and G.Z.; acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S.T., V.Y., and G.Z.; analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis): S.T. and G.Z.; writing, review, and/or revision of the manuscript: S.T., V.Y., and G.Z.; administrative, technical, or material support (ie, reporting or organizing data, constructing databases): S.T. and V.Y.; study supervision: G.Z.
References
- 1.Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl 5):v1–v75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 4.Thomas AA, Brennan CW, DeAngelis LM, Omuro AM. Emerging therapies for glioblastoma. JAMA Neurol. 2014;71(11):1437–1444. [DOI] [PubMed] [Google Scholar]
- 5.Petticrew M, Rehfuess E, Noyes J, et al. Synthesizing evidence on complex interventions: how meta-analytical, qualitative, and mixed-method approaches can contribute. J Clin Epidemiol. 2013;66(11):1230–1243. [DOI] [PubMed] [Google Scholar]
- 6.Welton NJ, Caldwell DM, Adamopoulos E, Vedhara K. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol. 2009;169(9):1158–1165. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay F, Jackson G, Rosiñol L, et al. Maintenance treatment and survival in patients with myeloma: a systematic review and network meta-analysis. JAMA Oncol. 2018;4(10):1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse JW, Schandelmaier S, Kamaleldin M, et al. Opioids for chronic non-cancer pain: a protocol for a systematic review of randomized controlled trials. Syst Rev. 2013;2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. 2020;16(1):e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 16.Herrlinger U, Schäfer N, Steinbach JP, et al. Bevacizumab plus irinotecan versus temozolomide in newly diagnosed O6-methylguanine-DNA methyltransferase nonmethylated glioblastoma: the randomized GLARIUS trial. J Clin Oncol. 2016;34(14):1611–1619. [DOI] [PubMed] [Google Scholar]
- 17.Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 18.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westphal M, Heese O, Steinbach JP, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522–532. [DOI] [PubMed] [Google Scholar]
- 20.Ursu R, Carpentier A, Metellus P, et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study. Eur J Cancer. 2017;73:30–37. [DOI] [PubMed] [Google Scholar]
- 21.Cho DY, Yang WK, Lee HC, et al. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: a phase II clinical trial. World Neurosurg. 2012;77(5–6):736–744. [DOI] [PubMed] [Google Scholar]
- 22.Wen PY, Reardon DA, Armstrong TS, et al. A randomized double-blind placebo-controlled phase 2 trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;18:5799–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang DY, Bu XY, Zhou ZL, et al. Enhanced antitumor effects of radiotherapy combined local nimustine delivery rendezvousing with oral temozolomide chemotherapy in glioblastoma patients. J Cancer Res Ther. 2018;14(1):78–83. [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi T, Natsume A, Mizusawa J, et al. JCOG0911 INTEGRA study: a randomized screening phase II trial of interferonβ plus temozolomide in comparison with temozolomide alone for newly diagnosed glioblastoma. J Neurooncol. 2018;138(3):627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallick S, Kunhiparambath H, Gupta S, et al. Hypofractionated accelerated radiotherapy (HART) with concurrent and adjuvant temozolomide in newly diagnosed glioblastoma: a phase II randomized trial (HART-GBM trial). J Neurooncol. 2018;140(1):75–82. [DOI] [PubMed] [Google Scholar]
- 26.Elinzano H, Glantz M, Mrugala M, et al. PPX and concurrent radiation for newly diagnosed glioblastoma without MGMT methylation: a randomized phase II study: BrUOG 244. Am J Clin Oncol. 2018;41(2):159–162. [DOI] [PubMed] [Google Scholar]
- 27.Chinnaiyan P, Won M, Wen PY, et al. A randomized phase II study of everolimus in combination with chemoradiation in newly diagnosed glioblastoma: results of NRG Oncology RTOG 0913. Neuro Oncol. 2018;20(5):666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchroithner J, Erhart F, Pichler J, et al. Audencel immunotherapy based on dendritic cells has no effect on overall and progression-free survival in newly diagnosed glioblastoma: a phase II randomized trial. Cancers. 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weller M, Butowski NA, Tran DD, et al. ACT IV: an international, double-blind, phase 3 trial of rindopepimut in newly diagnosed, EGFRvIII-expressing glioblastoma. Neuro Oncol. 2017;19(suppl 3):iii22. [DOI] [PubMed] [Google Scholar]
- 30.Kong DS, Nam DH, Kang SH, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8(4):7003–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wick W, Gorlia T, Bady P, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082). Clin Cancer Res. 2016;22(19):4797–4806. [DOI] [PubMed] [Google Scholar]
- 32.Penas-Prado M, Hess KR, Fisch MJ, et al. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro Oncol. 2015;17(2):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabors LB, Fink KL, Mikkelsen T, et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 2015;17(5):708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 37.Weller M, Butowski N, Tran DD, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 38.Stupp R, Hegi ME, Idbaih A, et al. Tumor treating fields added to standard chemotherapy in newly diagnosed glioblastoma (GBM): final results of a randomized, multi-center, phase III trial. Cancer Research. Conference: American Association for Cancer Research Annual Meeting.2017;77(13suppl 1). [Google Scholar]
- 39.Szopa W, Burley TA, Kramer-Marek G, Kaspera W. Diagnostic and therapeutic biomarkers in glioblastoma: current status and future perspectives. Biomed Res Int. 2017;2017:8013575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appay R, Dehais C, Maurage CA, et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019;21(12):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerstner ER, Duda DG, di Tomaso E, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol. 2009;6(4):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 43.Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.