At the outset of the coronavirus disease 2019 (COVID-19) pandemic, a few researchers [1, 2] postulated that patients with COVID-19 who are receiving renin–angiotensin system (RAS) inhibitors might be at an increased risk for a severe course of illness. This postulation was based on the fact that angiotensin-converting enzyme 2 (ACE2) is the target receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and RAS inhibitors can upregulate the levels of ACE2 and thus facilitate the entry of SARS-CoV-2 into human host cells. Subsequently, advocates proposed the temporary discontinuation of RAS inhibitors in patients with COVID-19 [1, 2]. Nevertheless, we argued that exposure to RAS inhibitors does not consistently lead to the upregulation of the levels of ACE2 and that the ability of RAS inhibitors to upregulate ACE2 is not absolutely deleterious, since, paradoxically, the upregulation of ACE2 might protect against coronavirus-induced acute lung injury [3–5]. Therefore, the discontinuation of RAS inhibitors in patients with COVID-19 is controversial, and consideration must be given to whether the established benefits of these agents with regard to cardiovascular diseases and their potential lung-protective properties outweigh their uncertain risks [3, 6–8]. Our recent meta-analysis of observational evidence [9] asserted that patients with COVID-19 who used RAS inhibitors had a significantly lower risk of mortality than those who did not use RAS inhibitors. It is likely that residual confounding might have existed in previous observational studies; therefore, further confirmation of such an association is needed in randomized controlled trials. We conducted this meta-analysis of randomized controlled trials to validate the association between the use of RAS inhibitors and mortality in patients with COVID-19.
A systematic literature search was performed in electronic databases, including PubMed, Scopus, the Cochrane Central Register of Controlled Trials, and preprint servers (medRxiv, Research Square, SSRN) with no language restriction to identify eligible studies published prior to March 14, 2021. The search strategy was built based on the following keywords and MeSH terms: “COVID-19”, “SARS-CoV-2”, “randomized controlled trials”, “angiotensin-converting enzyme”, “ACE”, “ACE inhibitor”, “angiotensin receptor blocker”, “ARB”, “renin–angiotensin-system”, “RAS inhibitor”, “renin–angiotensin–aldosterone”, “RAA inhibitor”, and “RAAS inhibitor”. The World Health Organization international clinical trial registry platform (who.int/clinical-trials-registry-platform) and the clinical trial registry of the United States (clinicaltrials.gov) were also searched to identify registered trials with reported findings. Two investigators (CSK and SSH) independently performed the literature screening to identify eligible studies. The reference lists of relevant articles were also reviewed to identify potentially eligible studies. The eligibility criteria for the inclusion of studies included a randomized controlled trial design and the comparison of mortality in patients with COVID-19 who did and did not use RAS inhibitors. We excluded studies with observational designs, nonrandomized trials, single-arm trials, and trials that did not report mortality outcomes.
The outcome of interest was all-cause mortality. Each included trial was independently evaluated by two investigators (CSK and SSH) who also extracted the study characteristics. The data collected included author(s), trial design, country, patient age, proportion of patients using RAS inhibitors, and mortality outcomes. Furthermore, to evaluate potential bias in the reports of randomized trials, two investigators (CSK and SSH) assessed the risk of bias in the included trials with a standardized method, namely, version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) [10]. Essentially, RoB 2 is structured into a fixed set of domains of bias, including different aspects of the trial design, conduct, and reporting. The random effects model was utilized for the meta-analysis to estimate the pooled odds ratio with 95% confidence intervals. Subsequently, the heterogeneity between studies was examined using the I2 statistic and the χ2 test, with cutoff values of 50% and P < 0.10, respectively. The meta-analysis was performed using Meta XL, version 5.3 (EpiGear International, Queensland, Australia).
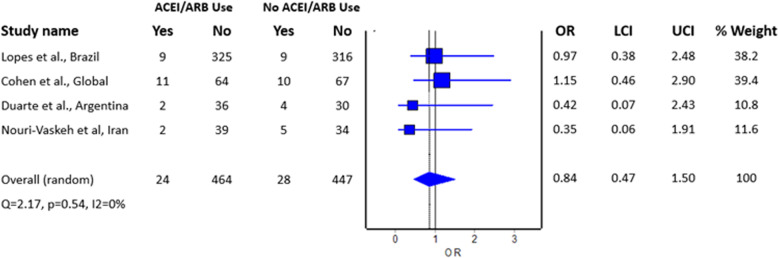
Our systematic literature search returned 878 titles, of which 440 were unique (titles retrieved after removing duplications). Four randomized controlled trials [11–14] were included after screening, with a total of 488 patients who were randomized to the use of RAS inhibitors during hospitalization for COVID-19 and 475 patients who were randomized to the control group and did not receive RAS inhibitors during hospitalization for COVID-19. Specifically, two trials [11, 12] investigated the continued use of RAS inhibitors versus the discontinuation of RAS inhibitors, while the other two trials [13, 14] evaluated the effect of the de novo introduction of RAS inhibitors (telmisartan and losartan, respectively) versus standard care and amlodipine, respectively, among patients hospitalized for COVID-19. Three of the included randomized trials in the meta-analysis were from Brazil [11], Argentina [13], and Iran [14], whereas the remaining randomized trial [12] was an international multicenter study performed in seven countries. Details of the included studies and the overall risk of bias assessed by RoB 2 are depicted in Table 1. In terms of the risk of bias, all the included trials had some concerns with regard to the overall risk of bias; the trials by Lopes et al. [11], Cohen et al. [12], and Duarte et al. [13] had some concerns over the risk of bias in the domain of “deviations from intended interventions” because of their open-label trial design, whereas the trial by Nouri-Vakesh et al. [14] had some concerns over the risk of bias in the domain of “selection of the reported results” due to the possibility that the trial was not anlyzed as pre-specified.
Table 1.
Characteristics of included trials
| Study, Ref. | Country | Design | Total number of patients | Age (median/mean unless otherwise specified) | Proportion of RAS inhibitors use in the treatment group (%) | Mortality | Risk of bias | |
|---|---|---|---|---|---|---|---|---|
| RAS inhibitors users n/N; %) |
Non-RAS inhibitors users (n/N; %) |
|||||||
| Lopes et al. [11] | Brazil | Randomized, open-label trial | 659 |
RAS inhibitor users = 56 (46.1–66.1) Non-RAS inhibitor users = 55 (46.1–63.1) |
ACE inhibitor = 21.0 ARB = 79.0 |
9/334 (2.7) | 9/325 (2.8) | Some concerns |
| Cohen et al. [12] | Global | Randomized, open-label trial | 152 |
RAS inhibitor users = 62 (12) Non-RAS inhibitor users = 62 (12) |
ACE inhibitor = 33.3 ARB = 66.7 |
11/75 (14.7) | 10/77 (13.0) | Some concerns |
| Duarte et al. [13] | Argentina | Randomized, open-label trial | 72 |
RAS inhibitor users = 63.8 (18.7) Non-RAS inhibitor users = 60.1 (17.8) |
ARB (telmisartan 80 mg twice daily for 14 days) = 100 | 2/38 (5.3) | 4/34 (11.8) | Some concerns |
| Nouri-Vaskeh et al. [14] | Iran | Randomized, double-blind, controlled trial | 80 |
RAS inhibitor users = 67.3 (14.8) Non-RAS inhibitor users = 60.1 (17.3) |
ARB (losartan 25 mg twice daily for at least 14 days) = 100 | 2/41 (4.9) | 5/39 (12.8) | Some concerns |
The meta-analysis revealed no difference in the risk of mortality between patients with COVID-19 who did and did not use RAS inhibitors; the estimated effect measure indicated no benefit with the use of RAS inhibitors with regard to mortality (Fig. 1; pooled odds ratio = 0.84; 95% confidence interval 0.47–1.50, n = 963), where there was inadequate evidence to refute the model hypothesis of “no significant difference” given the current sample size. Of note, these findings conflict with those of our previous meta-analysis [9] of observational studies, in which the use of RAS inhibitors was found to be associated with reduced mortality. The findings seem to suggest that while the downregulation of ACE2 upon the entry of SARS-CoV-2 into host cells can occur [15], it may not adequately explain for the underlying mechanism of mortality in patients with COVID-19 tested in the included studies. In addition, because individual RAS inhibitors have different effects on ACE2 expression, it may be difficult to determine the effect in trials that included patients who received any type of RAS inhibitor. Thus far, only the trial by Duarte et al. [13] and the trial by Nouri-Vakesh et al. [14] assessed the use of a single RAS inhibitor (telmisartan and losartan, respectively), although they reported no benefit with regard to mortality. Regardless of the absence of mortality benefits, the findings from our meta-analysis, for the first time, indicate the safety of RAS inhibitors among patients with COVID-19.
Fig. 1.
Pooled odds ratio for mortality in RAS inhibitor users compared to nonusers hospitalized with COVID-19
Compliance with ethical standards
Conflict of interest
The author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kow CS, Zaidi STR, Hasan SS. Cardiovascular disease and use of renin-angiotensin system inhibitors in COVID-19. Am J Cardiovasc Drugs. 2020;20:217–21. doi: 10.1007/s40256-020-00406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan CS, Yeoh SF, Long CM. COVID-19: critical role of angiotensin 1-7 in ACE2 modulation. Ann Acad Med Singap. 2020;49:398–400. doi: 10.47102/Annals-acadmedsg.202085. [DOI] [PubMed] [Google Scholar]
- 5.Shibata S, Arima H, Asayama K, Hoshide S, Ichihara A, Ishimitsu T, et al. Hypertension and related diseases in the era of COVID-19: a report from the Japanese Society of Hypertension Task Force on COVID-19. Hypertens Res. 2020;43:1028–46. doi: 10.1038/s41440-020-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narita K, Hoshide S, Tsoi K, Tsoi K, Siddique S, Shin J, et al. Disaster hypertension and cardiovascular events in disaster and COVID-19 pandemic. J Clin Hypertens. 2021 doi: 10.1111/jch.14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuzawa Y, Ogawa H, Kimura K, Konishi M, Kirigaya J, Fukui K, et al. Renin-angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43:1257–66. doi: 10.1038/s41440-020-00535-8. [DOI] [PubMed] [Google Scholar]
- 8.Kario K, Morisawa Y, Sukonthasarn A, Turana Y, Chia YC, Park S, et al. COVID-19 and hypertension-evidence and practical management: Guidance from the HOPE Asia Network. J Clin Hypertens. 2020;22:1109–19. doi: 10.1111/jch.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID-19 patients receiving renin-angiotensin system inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2020;20:571–90. doi: 10.1007/s40256-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.Lopes RD, Macedo AVS, de Barros E, Silva PGM, Moll-Bernardes RJ, Dos Santos TM, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–64. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte M, Pelorosso F, Nicolosi L, Salgado MV, Vetulli H, Aquieri A, et al. Telmisartan for treatment of Covid-19 patients: an open randomized clinical trial—a preliminary report. Preprint. medRxiv. 2020. 10.1101/2020.08.04.20167205.
- 14.Nouri-Vaskeh M, Kalami N, Zand R, Soroureddin Z, Varshochi M, Ansarin K, et al. Comparison of losartan and amlodipine effects on the outcomes of patient with COVID-19 and primary hypertension: a randomized clinical trial. Int J Clin Pract. 2021;e14124. online ahead of print. [DOI] [PMC free article] [PubMed]
- 15.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE2. Circ Res. 2021. 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed]