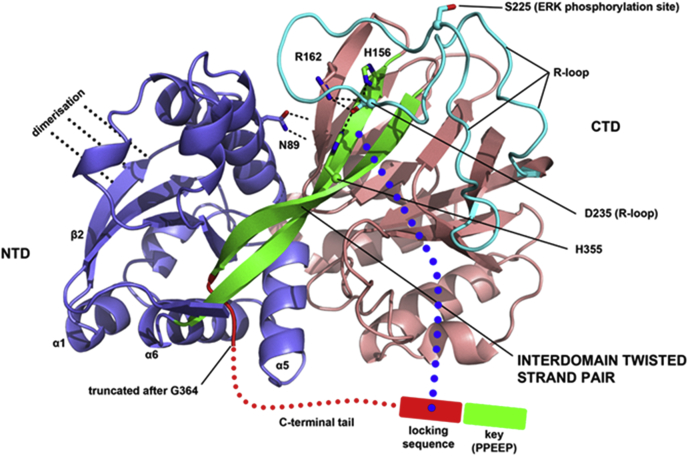
Figure 12.
Hypothesized structural rationale for alignment of membrane engagement determinants orchestrated by the R-loop and C-terminal tail. SK1 exhibits a long, twisted strand pair (green ribbon) that acts as a ‘connecting rod’ between the NTD (slate-blue ribbon) and CTD (salmon ribbon), illustrated here from the crystal structure of hSK1 (PDB ID: 4L02). Flex and twist about the connecting rod is postulated to control interdomain movement and thence topographical alignment or misalignment of key membrane-interfacing determinants—K27, K29, R186, LBL-1 hydrophobic surface (obscured in this view perspective)—for anionic phospholipid-enriched membrane engagement. The protein conformation exhibited in this crystal structure, where the C-terminal tail (red dotted line) has been removed after G364, likely corresponds to the active state alignment of the NTD and CTD and features interdomain hydrogen bonding from N89 and engagement of connecting rod histidines (H156/H355) by R-loop D235 (labeled) to stabilize the observed conformational arrangement of domains. In cytosol, with the intact C-terminal tail, a ‘locking sequence’ (residues 6–10 preceding the C terminus in mSK1) is postulated to obstruct adoption of the active state conformation and maintain the NTD and CTD membrane-interfacing determinants in a misaligned state. This may involve folding of the locking sequence onto the CTD (blue dotted line), potentially with rotation about the twisted pair. Gq signaling manipulates a ‘key sequence’ (PPEEP) at the C terminus, possibly by binding of an adapter protein, to remove the influence of the locking sequence and allow adoption of the conformation with aligned membrane-interfacing determinants. R-loop phosphorylation on S225 (labeled) by ERK also drives adoption of an active state conformation in a manner that does not require the presence of the C-terminal key. In principle, this may involve a structural transition in the R-loop that overturns the hypothesized folding of the locking sequence across the CTD and forces an active-state twist on the connecting rod. Alternatively, R-loop phosphorylation might facilitate protein recruitment to stabilize the active-state conformation and overturn the locking sequence. CTD, C-terminal domain; ERK, extracellular signal–regulated kinase; hSK1, human SK1; LBL-1, lipid-binding loop 1; mSK1, mouse SK1; NTD, N-terminal domain; R-loop, regulatory loop; SK1, sphingosine kinase 1.