Figure 1.
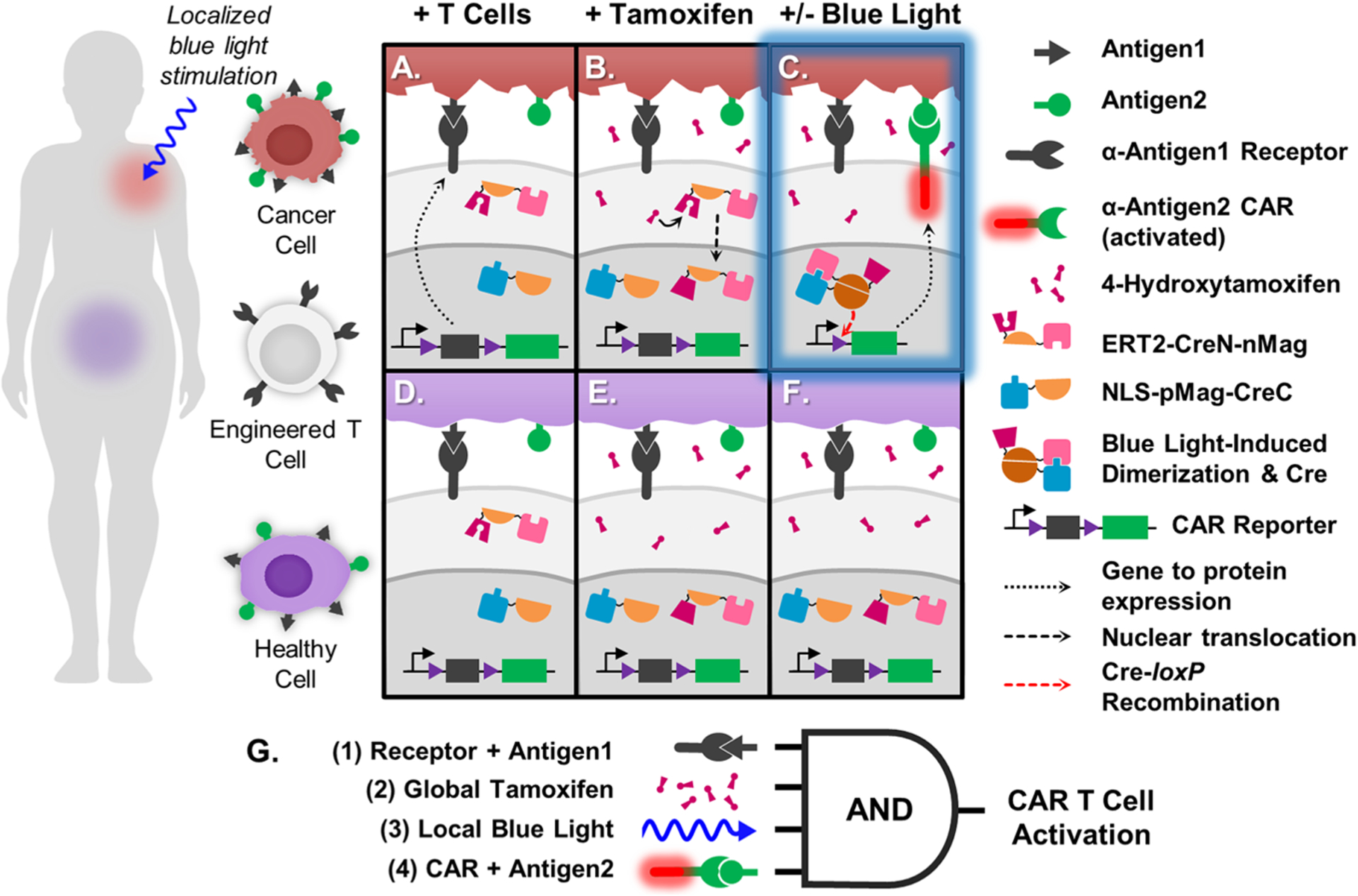
Schematic of TamPA-Cre application and molecular mechanism. (Left) Person with Antigen1+ Antigen2+ cancerous (red) and healthy (purple) tissues in separate regions of the body. Engineered T cells express TamPA-Cre (ERT2-CreN-nMag and NLS-pMag-CreC) and the CAR Reporter genetic construct, consisting of a constitutive promoter driving expression of the floxed (purple) α-Antigen1 Receptor CDS with stop codons (black), followed by α-Antigen2 CAR (green). Upon intravenous introduction, the engineered T cells bind and localize to both cancerous (A) and healthy (D) Antigen1+ cells. TamPA-Cre is inactive as its NLS-pMag-CreC and ERT2-CreN-nMag protein halves nuclear and cytosolically localized, respectively. After administration of tamoxifen, metabolite 4-OHT binds with ERT2-CreN-nMag to drive nuclear localization (B,E), priming TamPA-Cre. Next, blue light is applied to the cancerous tissue region only (C), inducing nMag-pMag heterodimerization which restores active TamPA-Cre recombinase activity within the nucleus. The floxed α-Antigen1 Receptor CDS in the CAR Reporter is excised through Cre-loxP recombination along with its stop codons, thus allowing for α-Antigen2 CAR expression. The T cell is finally activated upon CAR-mediated binding to Antigen2. T cells localized to the healthy tissue region (F) are not exposed to blue light and thus do not express α-Antigen2 CAR, effectively protecting healthy cells that express both Antigen1 and Antigen2. (G) Boolean logic representation of the AND-gated TamPA-Cre system. (1) T cells first bind to cells expressing Antigen1 via the Receptor. (2) Then, TamPA-Cre is be primed with tamoxifen before receiving localized (3) blue light stimulation in the cancerous tissue region, driving Cre-loxP recombination and CAR expression. (4) T cell activation is triggered when Antigen2 is recognized by the CAR.
