Figure 4.
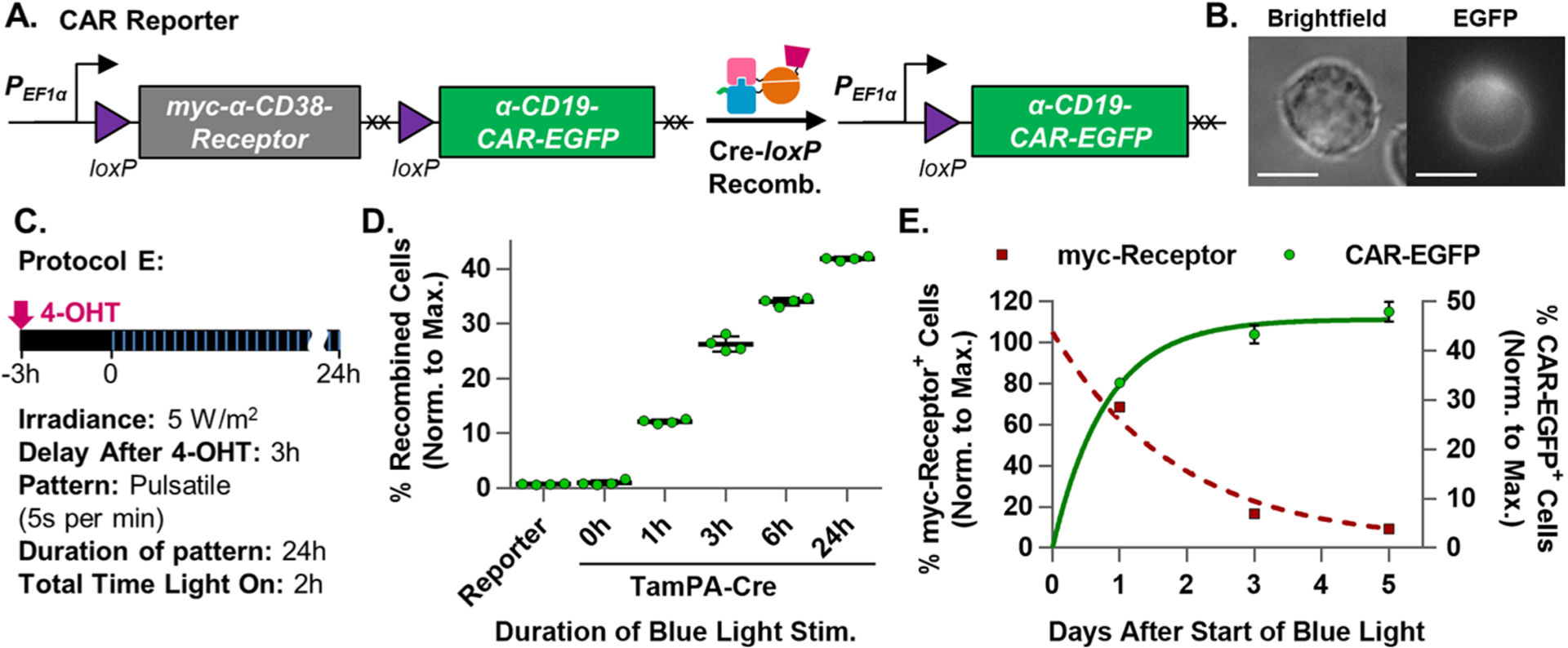
Optimization and characterization of the TamPA-Cre system in Jurkat T cells. (A) Schematic of the CAR Reporter construct before and after TamPA-Cre-mediated Cre-loxP recombination. The hEF1α promoter initially drives myc-α-CD38-Receptor expression. During Cre-loxP recombination, the floxed myc-α-CD38Receptor (with its stop codons, XX) is irreversibly excised allowing for (B) α-CD19CAR-EGFP expression (Jurkat T cell, 100×, scale bar = 10 μm). (C) Schematic illustrating tamoxifen (500 nM) and blue light stimulation (473 ± 29 nm) Protocol E: (5 W/m2, 5 s per min, 24 h) started 3 h after 4-OHT addition. (D) The percentage of recombined TamPA-Cre+ CAR Reporter Jurkat T cells (normalized to maximal recombination) exposed to Protocol E over the course of 0, 1, 3, 6, or 24 h (n = 4). (E) Normalized percentage of myc-Receptor+ and CAR-EGFP+ TamPA-Cre+ CAR Reporter Jurkat T cells stimulated by Protocol E, measured 1, 3, and 5 days after start of blue light stimulation, fitted with exponential decay and association trendlines (GraphPad, Table S2) (n = 4). Reporter = CAR Reporter Jurkat T cell line. CAR-EGFP flow cytometry measurements taken 72 h after the start of blue light stimulation. Percentage of recombined (% CAR-EGFP+) Jurkat T cells (normalized to maximal recombination) = 100%*(% of CAR-EGFP+ cells)/(initial % of CAR Reporter+ cells, measured via myc).
