Figure 4. Mechanisms of protein persulfidation.
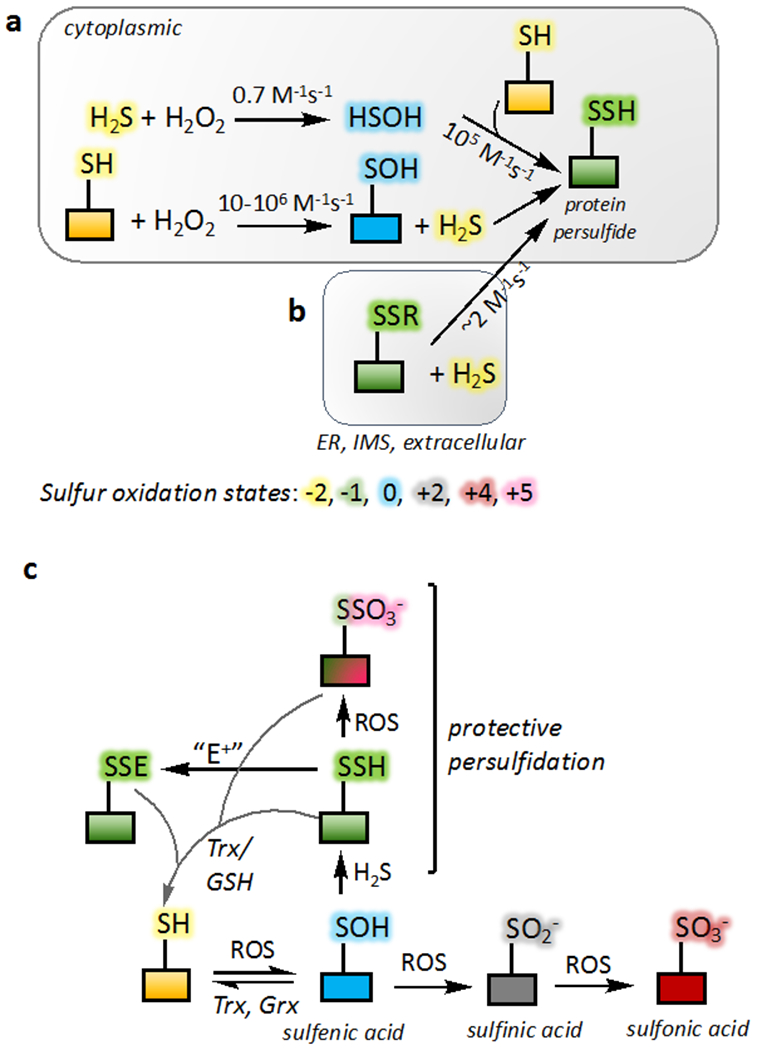
(a) Protein persulfidation requires an initial oxidation of the protein thiol or H2S. The bimolecular rate constant for the reaction of HSOH with free (105 M−1s−1) versus protein thiols is noted. (b) In oxidizing compartments within the cell such as the endoplasmic reticulum (ER) and the intermitochondrial membrane space (IMS), H2S can add into protein disulfides. The rate constant shown is for the reaction of H2S with a mixed disulfide between human serum albumin and GSH (Cuevasanta et al., 2015). (c) The irreversibility of some oxidized thiol modifications (sulfinic and sulfonic acid) can be averted via protein persulfidation at the level of sulfenic acid. Similarly, persulfide modification by an electrophile (E+) is reversible unlike modification of a thiol. The products can be reduced back to the thiol form by a disulfide reductases like thioredoxin (Trx) and glutaredoxin (Grx) or by GSH.
