Figure 8.
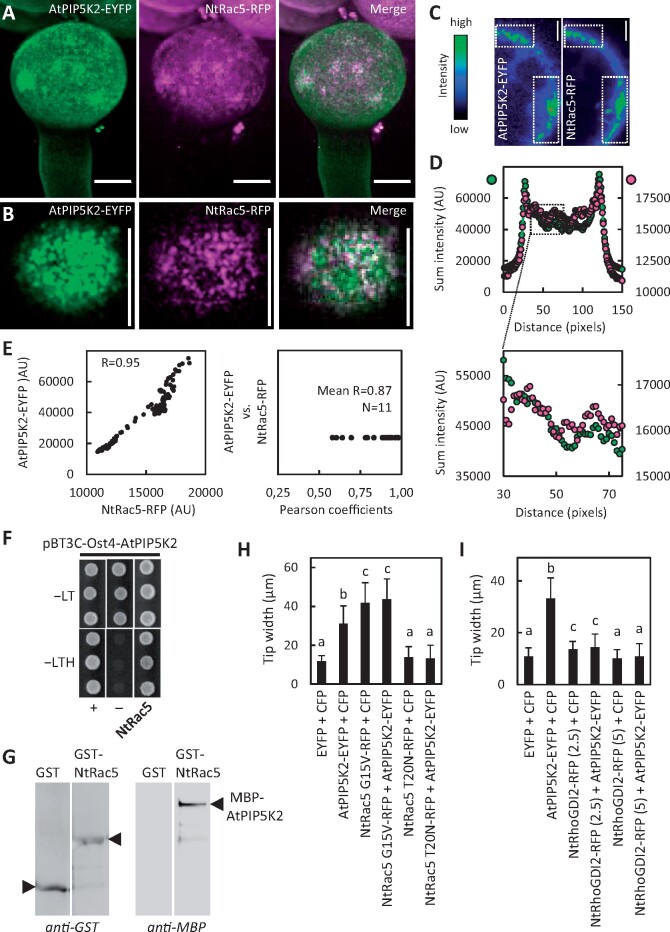
Coordinated localization, interaction and functional interplay of AtPIP5K2 with the actin regulator NtRac5. To explore the molecular mechanism by which AtPIP5K2 might influence actin dynamics, we tested for links to elements of ROP signaling, which are known to influence pollen tube actin. AtPIP5K2-EYFP was coexpressed with the tobacco ROP, NtRAC5-RFP, and fluorescence distributions were monitored by SD. A, Representative 3D reconstruction from z-stack acquisition (0.3-µm slices) of a pollen tube coexpressing AtPIP5K2-YFP and NtRAC5-RFP, as indicated. Scale bars, 10 µm. B, Fluorescence distribution at the cell surface of the pollen tube shown in (A), as indicated. Note that plasma membrane nanodomains of both AtPIP5K2-EYFP and NtRAC5-RFP are visible as bright dots at the cell surface. Scale bars, 10 µm. C, Representative median confocal frames extracted from the z-stack projection shown in (A) and representing the individual channels for AtPIP5K2-EYFP or NtRac5-RFP with heatmap colors, as indicated. Note that high intensity values from both channels localize in the same areas at the cell periphery. D, The sum intensities were determined for the vertical pixel columns of each channel (please refer to Supplementary Figure S5 and Materials and methods section for details). Upper panel, sum intensities for AtPIP5K2-YFP (green) and NtRac5-RFP (red). Lower panel, detail view if the region of interest indicated by the box in the upper panel. E, Correlation analysis of sum intensity profiles obtained for AtPIP5K2-EYFP and NtRac5-RFP. Left panel, Representative correlation plot for the fluorescence distributions given in (D). The correlation is characterized by a high Pearson coefficient R of 0.95. Right panel, Pearson coefficients obtained accordingly from 11 correlation analyses, with a mean R of 0.87, indicating consistently high correlations between the spatial distributions of AtPIP5K2-EYFP and NtRac5-RFP. F, G, AtPIP5K2 and NtRac5 proteins were tested for interaction in a protein complex. F, Interaction of AtPIP5K2 and NtRac5 according to split-ubiquitin-based yeast-two-hybrid analysis. In this system, AtPIP5K2 is expressed as a fusion to an Ost4-membrane anchor, which attaches the fusion protein to the cytoplasmic face of the endoplasmic reticulum. Positive interaction of the protein partners is indicated by yeast growth on selective −LTH media compared to growth of the positive (+) and negative controls (−). Growth of all colonies on −LT media indicates the presence of the correct genotypes of the transformed yeast tested. The data are shown in triplicates and are representative for four biological experiments with similar results. G, Interaction of AtPIP5K2 and NtRac5 according to immuno pull-down experiments. The AtPIP5K2 and NtRac5 proteins were recombinantly expressed in E. coli as fusions to N-terminal GST or MBP tags, respectively. The GST-NtRac5 and MBP-AtPIP5K2 proteins were affinity-purified and used for the pull-down experiment. Purified GST protein alone served as a negative control. Positive interaction is indicated by the detection of full-length MBP-AtPIP5K2 upon immunoprecipitation with GST-NtRac5, but not with GST alone. The blots shown are representative for two experiments. H, I, Pollen tube tip swelling was used as a morphological marker to assess the functional interplay of AtPIP5K2 with elements of tobacco ROP signaling in vivo. H, Functional interplay between AtPIP5K2 and NtRac5. Pollen tube tip widths were determined upon the coexpression of the control proteins, CFP with EYFP; AtPIP5K2-EYFP with CFP; the constitutive active NtRac5 G15V-RFP with CFP; AtPIP5K2-EYFP with NtRac5 G15V-RFP; the dominant negative NtRac5 T20N-RFP with CFP; and AtPIP5K2-EYFP with NtRac5 T20N-RFP. Please note the absence of pollen tube tip swelling upon the coexpression of AtPIP5K2-EYFP together with NtRac5 T20N-RFP, indicating that activation of NtRac5 is required for the effect of AtPIP5K2-EYFP on tip swelling. Data are given as means ± standard deviation and represent measurements of ≥100 transgenic pollen tubes. I, Functional interplay between AtPIP5K2 and NtRhoGDI2. Pollen tube tip widths were determined upon the coexpression of the control proteins, CFP with EYFP; AtPIP5K2-EYFP with CFP; NtRhoGDI2-RFP with CFP; and AtPIP5K2-EYFP with NtRhoGDI2-RFP. Numbers indicate the use of 2.5-µg or of 5-µg plasmid DNA for the biolistic transformation of pollen tubes to modulate the expression levels of NtRhoGDI2-RFP. Please note the reduced pollen tube tip swelling upon the coexpression of AtPIP5K2-EYFP together with increasing levels of NtRhoGDI2. Data are given as means ± standard deviation and represent measurements of ≥100 transgenic pollen tubes. Letters indicate significant differences according to a one-way Anova test with Tukey post hoc analysis.