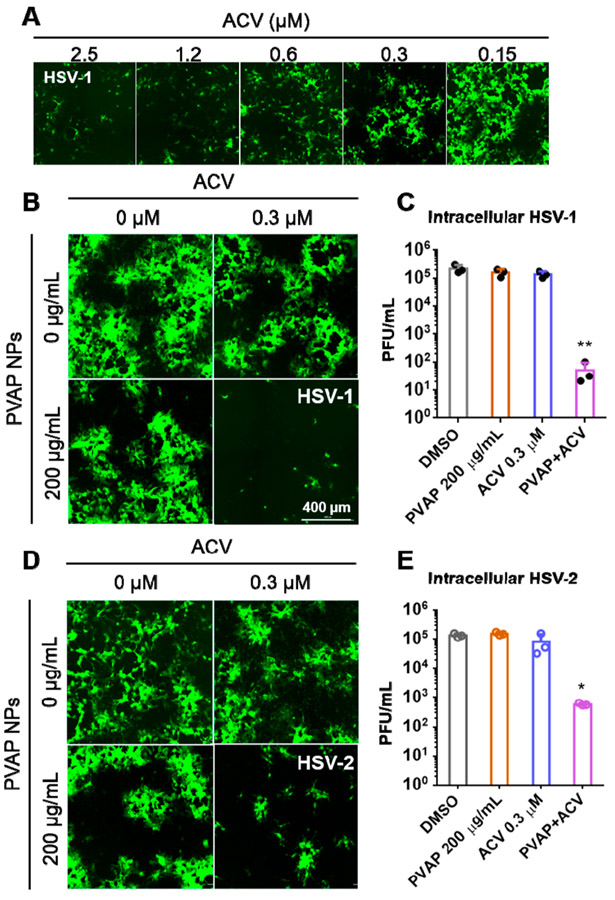
Figure 7.
Antiviral synergy evaluation of PVAP nanoparticles (NPs) with ACV. (A) HCEs were infected with 0.1 MOI GFP-expressing HSV-1 and then treated with shown concentrations of ACV. At 24 hpi, fluorescent images were recorded to evaluate the extent of infection (GFP spread). (B) Using the ACV (0.3 μM), 200 μg/mL PVAP NPs, and both of them in combination, HSV-1 infected HCE cells were therapeutically treated for a period of 24 h. At 24 hpi, fluorescent images were captured to evaluate the extent of viral spread. (C) Whole cell lysates were titrated for viral load using plaque assays. (D) In a similar experiment, HSV-2 infected HeLa cells were therapeutically treated with ACV (0.3 μM), 200 μg/mL PVAP NPs, and both of them in combination. At 24 hpi, fluorescent images were captured to evaluate the extent of viral spread. (E) Whole cell lysates were titrated for viral load using plaque assays. Asterisks indicate significance by multiple Student’s t test: *p < 0.05, **p < 0.01.