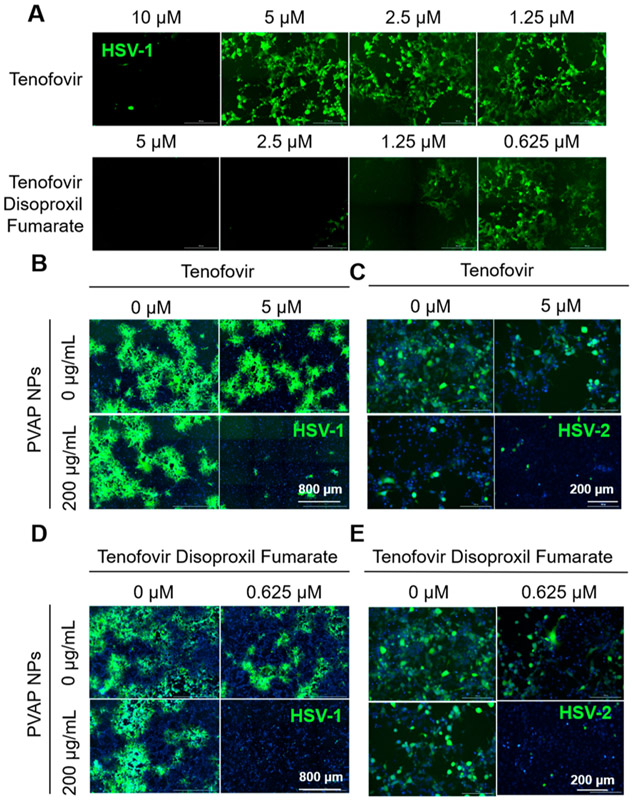
Figure 8.
Antiviral synergy of PVAP nanoparticles (NPs) with tenofovir and tenofovir disoproxil fumarate (TDF). (A) HCEs were infected with 0.1 MOI GFP-expressing HSV-1 and then treated with shown concentrations of tenofovir or TDF. At 24 hpi, fluorescent images were recorded to evaluate the extent of infection (GFP spread). Using 200 μg/mL PVAP NPs and tenofovir (5 μM) individually or in combination, HSV-1 infected HCE cells (B) or HSV-2 infected HeLa cells (C) were therapeutically treated for a period of 24 h. At 24 hpi, fluorescent images were captured to evaluate the extent of viral spread. In a similar experiment, 200 μg/mL PVAP NPs were synergized with TDF (0.625 μM) and tested on HSV-1 infected HCE cells (D) or HSV-2 infected HeLa cells (E). Experiments exploring the synergistic interaction among PVAP NPs and tenofovir/TDF against HSV-2 represented in 8 (C) and 8 (E) were carried out using same “untreated control” (“0 μM” in tenofovir, “0 μg/mL” PVAP NPs; “0 μM” tenofovir disoproxil fumarate, “0 μg/mL” PVAP NPs). Hence, the same representative image has been used for the untreated control.