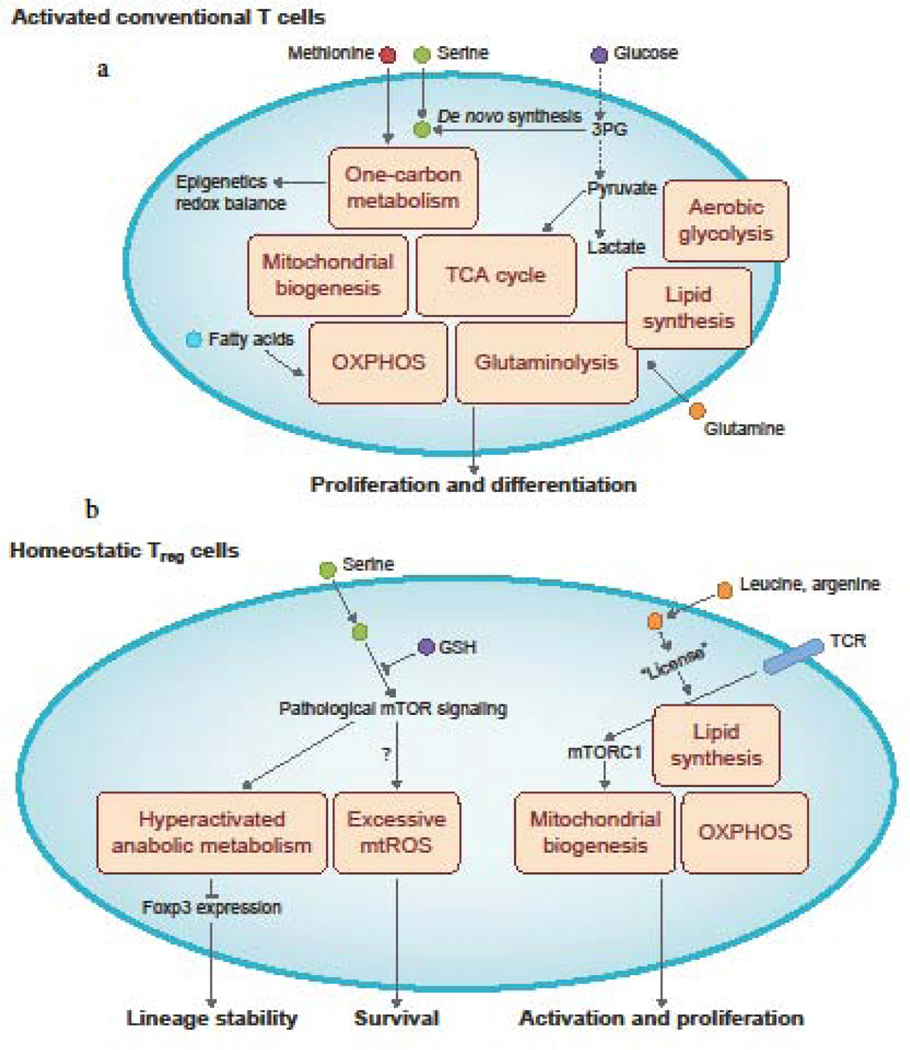
Figure 1: Cellular metabolism of activated conventional T cells and homeostatic Treg cells.
a. Upon their activation, conventional T cells upregulate the metabolic pathways of aerobic glycolysis, glutaminolysis, and lipid synthesis. They also increase mitochondria biogenesis to upregulate mitochondria-associated metabolic processes, including oxidative phosphorylation (OXPHOS), the tricarboxylic acid (TCA) cycle, and the methionine- and serine-dependent one-carbon metabolism pathway that is necessary for epigenetic programming and redox balance. Extracellular nutrients, including glucose, glutamine, methionine, and serine, are crucial for the induction of these different metabolic programs. Glucose metabolism can also support serine synthesis via its metabolic 3-phosphoglycerate (3PG). Fatty acids can also be used as a fuel source for OXPHOS in subsets of activated T cells. b. Mitochondrial biogenesis and OXPHOS, as well as lipid synthesis, are crucial for maintaining Treg cell functional activation and proliferation. These metabolic processes are activated downstream of mechanistic target of rapamycin complex 1 (mTORC1) signaling, which is induced by TCR engagement in the presence of the amino acids, leucine and arginine. Notably, excessive mitochondrial ROS (mtROS) production reduces Treg cell survival. Furthermore, excessive levels of anabolic metabolism (e.g. mitochondrial oxidative respiration or aerobic glycolysis), as a consequence of increased mTOR signaling, dampens Treg cell lineage stability. Treg cell stability can be counteracted by certain metabolites, including glutathione (GSH) that limits serine uptake and serine-induced mTORC1 activation in Treg cells.